EX-99.2
Published on September 9, 2020

FolRα – Targeting ADC Phase 1 Potential Best-in-Class ADC for Ovarian and Endometrial Cancers STRO 002 Exhibit 99.2
Forward Looking Statements This presentation and the accompanying oral presentation contain “forward-looking” statements that are based on our management’s beliefs and assumptions and on information currently available to management. Forward-looking statements include all statements other than statements of historical fact contained in this presentation, including information concerning our future financial performance, business plans and objectives, current and future clinical and preclinical activities, timing and success of our ongoing and planned clinical trials and related data, the timing of announcements, updates and results of our clinical trials and related data, timing and success of our planned development activities, our ability to obtain and maintain regulatory approval, the potential therapeutic benefits and economic value of our product candidates, potential growth opportunities, financing plans, competitive position, industry environment and potential market opportunities. Forward-looking statements are subject to known and unknown risks, uncertainties, assumptions and other factors, including risks and uncertainties related to our cash forecasts, our and our collaborators’ ability to advance our product candidates, the receipt and timing of potential regulatory submissions, designations, approvals and commercialization of product candidates, the timing and results of preclinical and clinical trials, and the expected impact of the COVID-19 pandemic on our operations. It is not possible for our management to predict all risks, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements we may make. These factors, together with those that may be described in greater detail under the heading “Risk Factors” contained in our most recent Annual Report on Form 10-K, Quarterly Report on Form 10-Q and other reports the company files from time to time with the Securities and Exchange Commission, may cause our actual results, performance or achievements to differ materially and adversely from those anticipated or implied by our forward-looking statements. You should not rely upon forward-looking statements as predictions of future events. Although our management believes that the expectations reflected in our forward-looking statements are reasonable, we cannot guarantee that the future results, levels of activity, performance or events and circumstances described in the forward-looking statements will be achieved or occur. Moreover, neither we nor our management assume responsibility for the accuracy and completeness of the forward-looking statements. We undertake no obligation to publicly update any forward-looking statements for any reason after the date of this presentation to conform these statements to actual results or to changes in our expectations, except as required by law. This presentation also contains estimates and other statistical data made by independent parties and by us relating to market size and growth and other data about our industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. In addition, projections, assumptions, and estimates of our future performance and the future performance of the markets in which we operate are necessarily subject to a high degree of uncertainty and risk. This presentation shall not constitute an offer to sell or the solicitation of an offer to buy, nor shall there be any sale of these securities in any state or other jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such state or other jurisdiction.
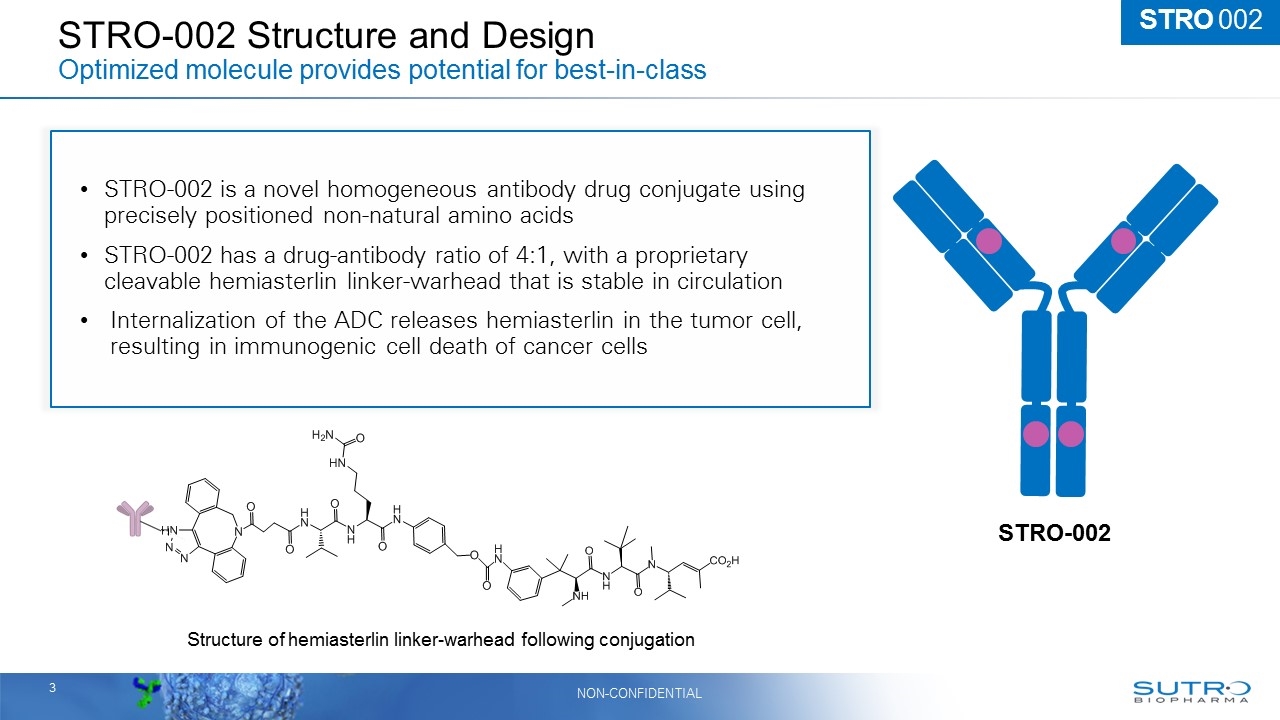
STRO-002 Structure and Design Optimized molecule provides potential for best-in-class STRO 002 STRO-002 Structure of hemiasterlin linker-warhead following conjugation STRO-002 is a novel homogeneous antibody drug conjugate using precisely positioned non-natural amino acids STRO-002 has a drug-antibody ratio of 4:1, with a proprietary cleavable hemiasterlin linker-warhead that is stable in circulation Internalization of the ADC releases hemiasterlin in the tumor cell, resulting in immunogenic cell death of cancer cells

Phase 1 Dose-Escalation Data Presented by Dr. Wendel Naumann

International Gynecologic Cancer Society Annual Meeting (Sept 10–13, 2020) Phase 1 Dose-Escalation Study of STRO-002, an anti-Folate Receptor alpha (FRα) Antibody Drug Conjugate (ADC), in Patients with Advanced Platinum-Resistant / Refractory Epithelial Ovarian Cancer (OC) R. Wendel Naumann1, Fadi S. Braiteh2, John P. Diaz3, Erika Hamilton4, Sami Diab5, Russell J. Schilder6, John W. Moroney7, Lainie P. Martin8, Denise Uyar9, David M. O'Malley10, Richard Penson11, Clifford DiLea12, Michael Palumbo13, Venita DeAlmeida13, Craig J. Berman13, Shannon Matheny13, Arturo Molina13 1Levine Cancer Institute, Carolinas Medical Center, Charlotte, NC; 2Comprehensive Cancer Centers of Nevada, Las Vegas, NV; 3Miami Cancer Institute at Baptist Health, Miami, FL; 4Sarah Cannon Research Institute, Tennessee Oncology PLLC, Nashville, TN; 5Rocky Mountain Cancer Center, Aurora, CO; 6Sidney Kimmel Cancer Center, Thomas Jefferson University, Philadelphia, PA; 7University of Chicago, Chicago, IL; 8University of Pennsylvania, Abramson Cancer Center, Philadelphia, PA; 9Medical College of Wisconsin, Milwaukee, WI; 10Ohio State University, Wexner Medical Center, Columbus, OH; 11 Massachusetts General Hospital, Boston, MA; 12Aclairo Pharmaceutical Development Group, Vienna, VA; 13Sutro Biopharma, Inc., South San Francisco, CA
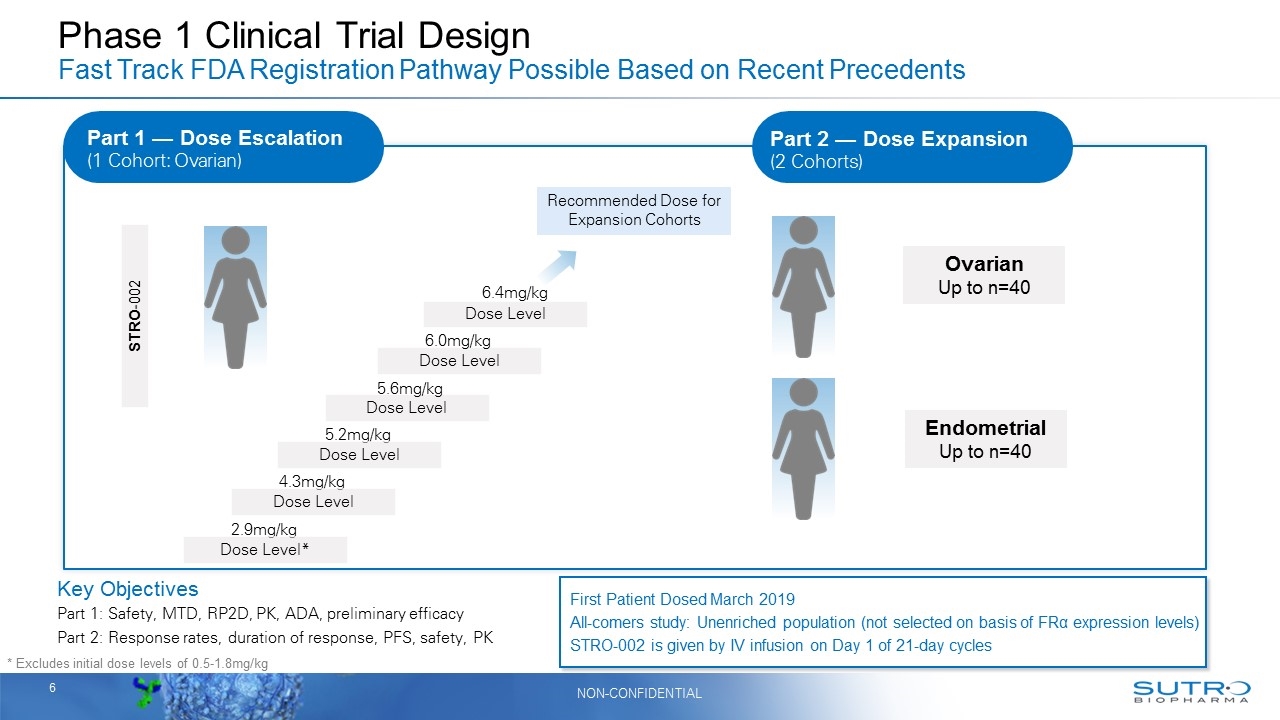
Phase 1 Clinical Trial Design Fast Track FDA Registration Pathway Possible Based on Recent Precedents Key Objectives Part 1: Safety, MTD, RP2D, PK, ADA, preliminary efficacy Part 2: Response rates, duration of response, PFS, safety, PK STRO-002 Dose Level Dose Level Dose Level 5.2mg/kg 5.6mg/kg 6.4mg/kg Recommended Dose for Expansion Cohorts Endometrial Up to n=40 Ovarian Up to n=40 * Excludes initial dose levels of 0.5-1.8mg/kg Dose Level 6.0mg/kg 4.3mg/kg Dose Level 2.9mg/kg Dose Level* First Patient Dosed March 2019 All-comers study: Unenriched population (not selected on basis of FRα expression levels) STRO-002 is given by IV infusion on Day 1 of 21-day cycles Part 2 — Dose Expansion (2 Cohorts) Part 1 — Dose Escalation (1 Cohort: Ovarian)
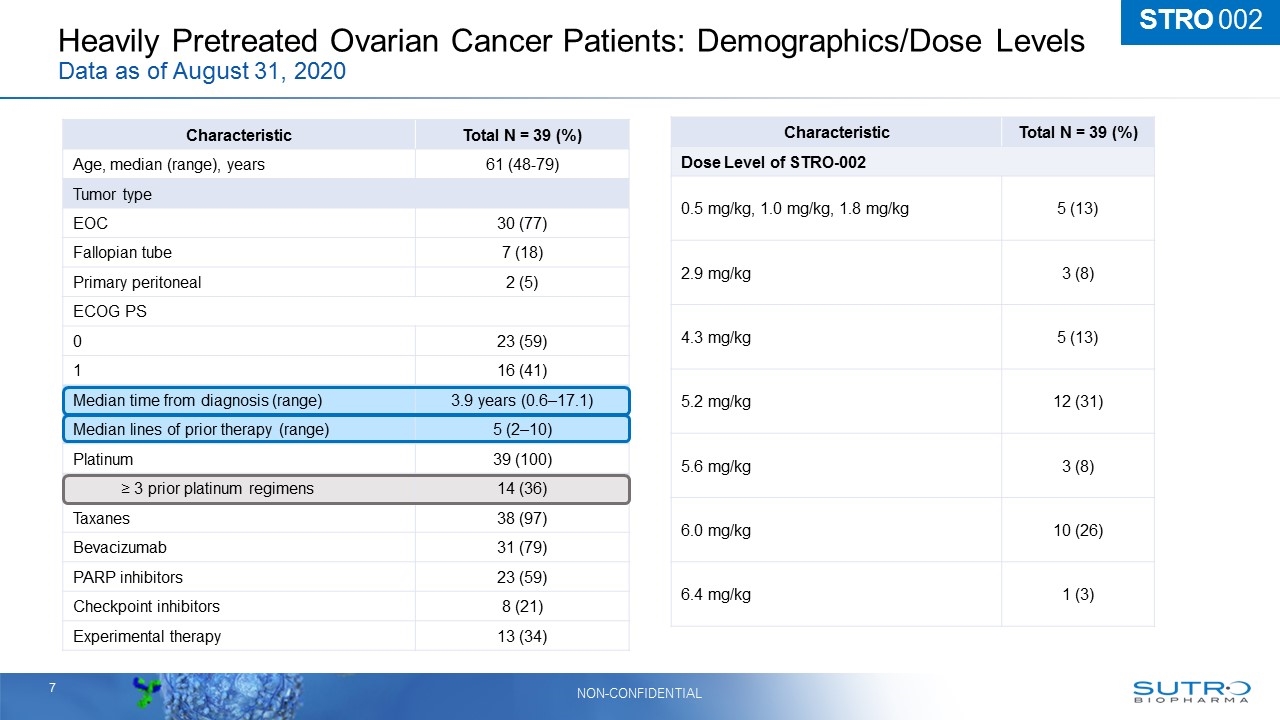
Heavily Pretreated Ovarian Cancer Patients: Demographics/Dose Levels Data as of August 31, 2020 Characteristic Total N = 39 (%) Age, median (range), years 61 (48-79) Tumor type EOC 30 (77) Fallopian tube 7 (18) Primary peritoneal 2 (5) ECOG PS 0 23 (59) 1 16 (41) Median time from diagnosis (range) 3.9 years (0.6–17.1) Median lines of prior therapy (range) 5 (2–10) Platinum 39 (100) ≥ 3 prior platinum regimens 14 (36) Taxanes 38 (97) Bevacizumab 31 (79) PARP inhibitors 23 (59) Checkpoint inhibitors 8 (21) Experimental therapy 13 (34) Characteristic Total N = 39 (%) Dose Level of STRO-002 0.5 mg/kg, 1.0 mg/kg, 1.8 mg/kg 5 (13) 2.9 mg/kg 3 (8) 4.3 mg/kg 5 (13) 5.2 mg/kg 12 (31) 5.6 mg/kg 3 (8) 6.0 mg/kg 10 (26) 6.4 mg/kg 1 (3) STRO 002
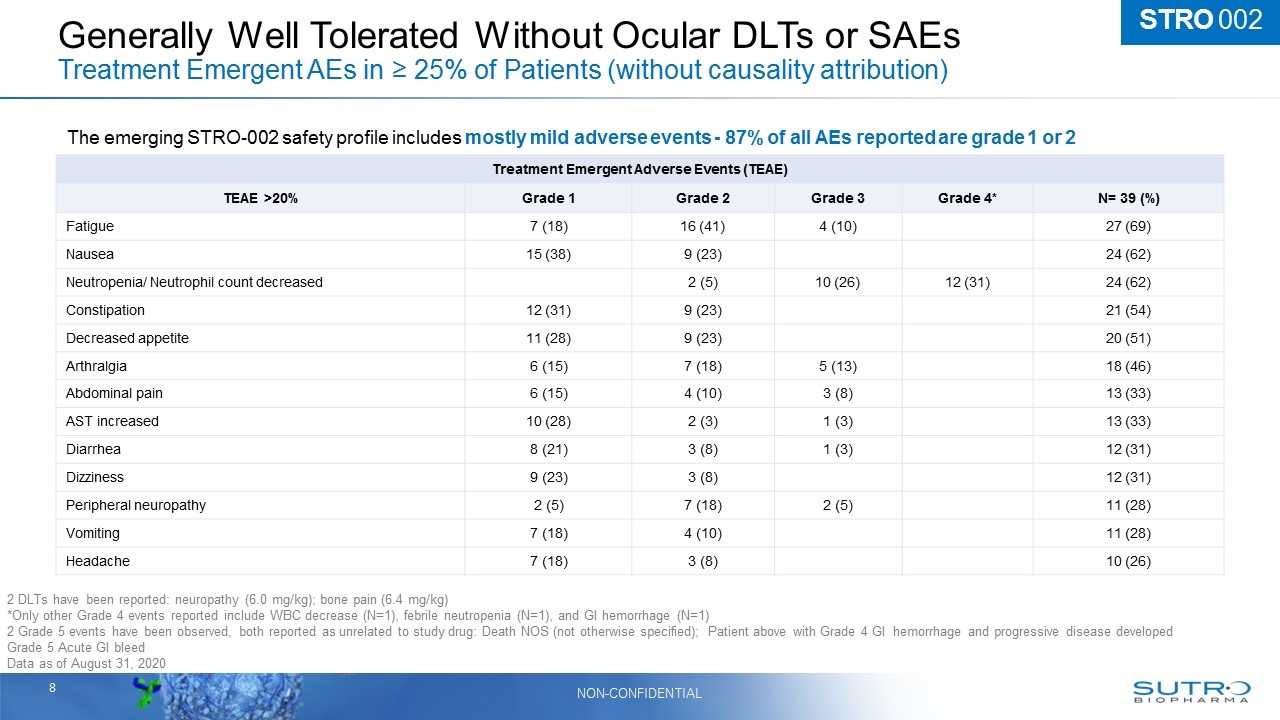
Generally Well Tolerated Without Ocular DLTs or SAEs Treatment Emergent AEs in ≥ 25% of Patients (without causality attribution) Treatment Emergent Adverse Events (TEAE) TEAE >20% Grade 1 Grade 2 Grade 3 Grade 4* N= 39 (%) Fatigue 7 (18) 16 (41) 4 (10) 27 (69) Nausea 15 (38) 9 (23) 24 (62) Neutropenia/ Neutrophil count decreased 2 (5) 10 (26) 12 (31) 24 (62) Constipation 12 (31) 9 (23) 21 (54) Decreased appetite 11 (28) 9 (23) 20 (51) Arthralgia 6 (15) 7 (18) 5 (13) 18 (46) Abdominal pain 6 (15) 4 (10) 3 (8) 13 (33) AST increased 10 (28) 2 (3) 1 (3) 13 (33) Diarrhea 8 (21) 3 (8) 1 (3) 12 (31) Dizziness 9 (23) 3 (8) 12 (31) Peripheral neuropathy 2 (5) 7 (18) 2 (5) 11 (28) Vomiting 7 (18) 4 (10) 11 (28) Headache 7 (18) 3 (8) 10 (26) The emerging STRO-002 safety profile includes mostly mild adverse events - 87% of all AEs reported are grade 1 or 2 STRO 002 2 DLTs have been reported: neuropathy (6.0 mg/kg); bone pain (6.4 mg/kg) *Only other Grade 4 events reported include WBC decrease (N=1), febrile neutropenia (N=1), and GI hemorrhage (N=1) 2 Grade 5 events have been observed, both reported as unrelated to study drug: Death NOS (not otherwise specified); Patient above with Grade 4 GI hemorrhage and progressive disease developed Grade 5 Acute GI bleed Data as of August 31, 2020
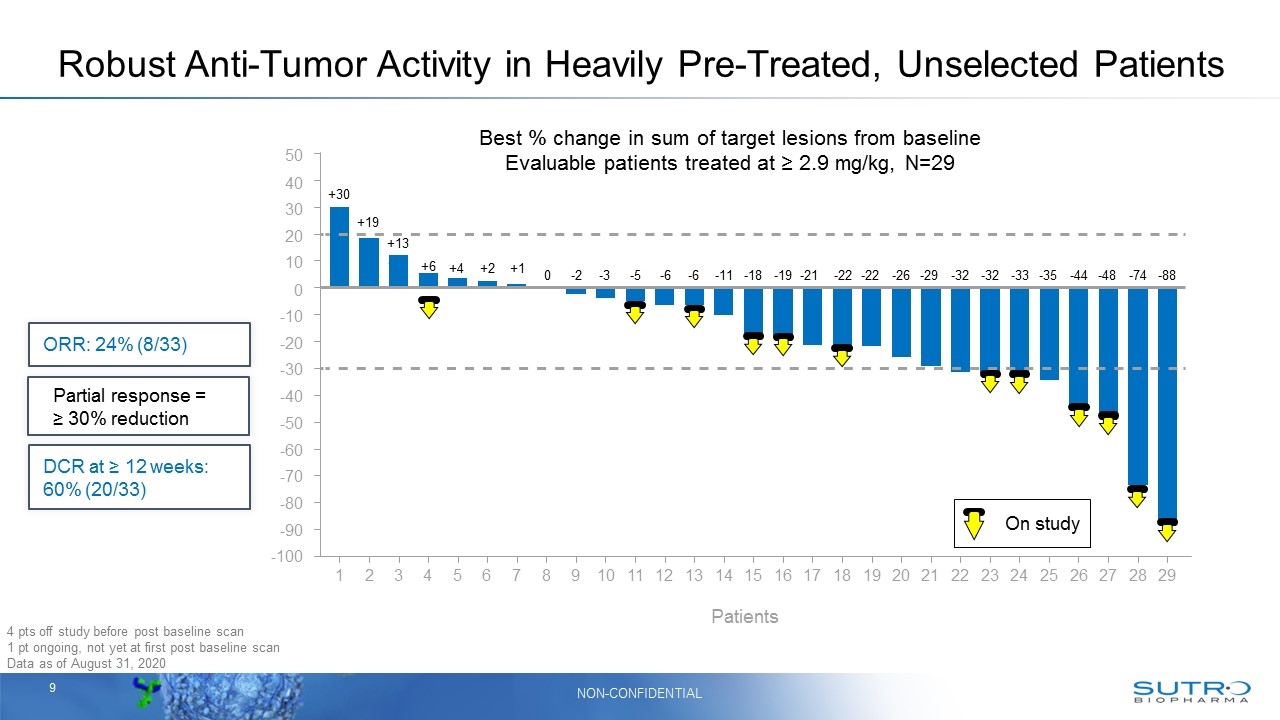
Best % change in sum of target lesions from baseline Evaluable patients treated at ≥ 2.9 mg/kg, N=29 4 pts off study before post baseline scan 1 pt ongoing, not yet at first post baseline scan Data as of August 31, 2020 Robust Anti-Tumor Activity in Heavily Pre-Treated, Unselected Patients +30 +6 +19 +13 +4 +2 +1 0 -2 -3 -5 -6 -6 -11 -18 -19 -21 -22 -22 -26 -29 -32 -32 -33 -35 -44 -48 -74 -88 Patients 50 40 30 20 10 0 -10 -20 -30 -40 -50 -60 -70 -80 -90 -100 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 On study +6 ORR: 24% (8/33) DCR at ≥ 12 weeks: 60% (20/33) Partial response = ≥ 30% reduction
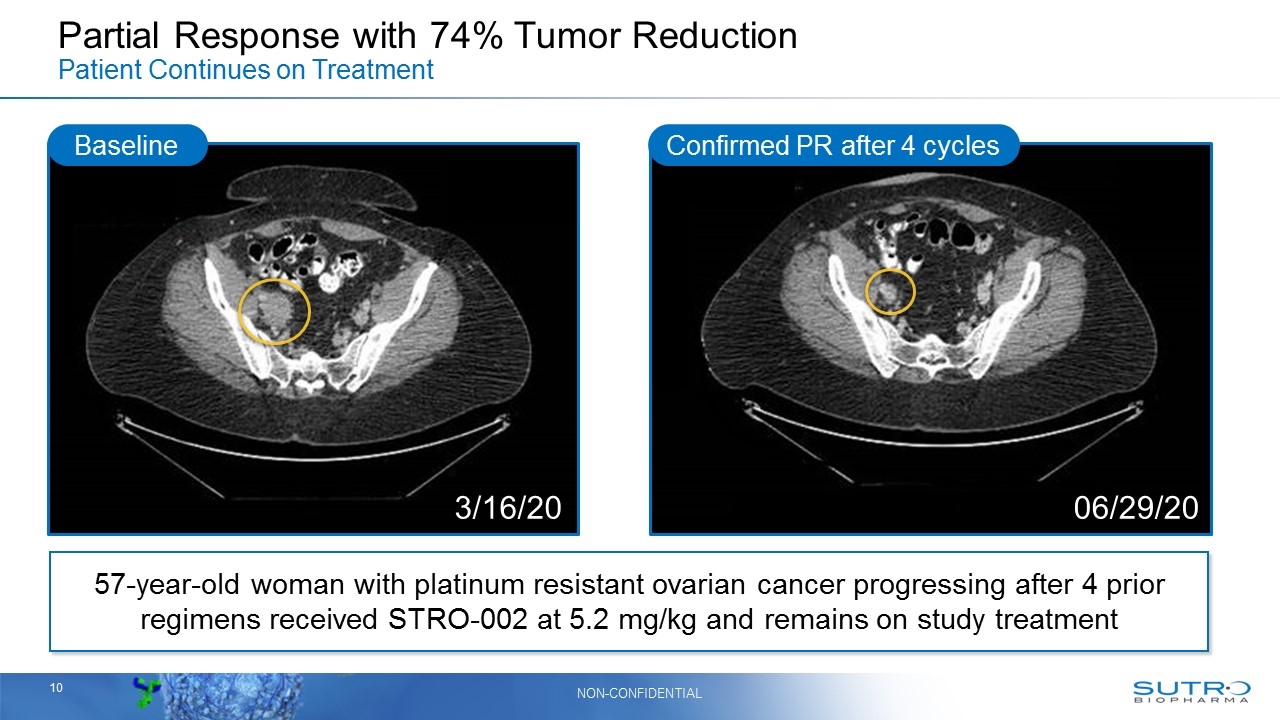
Partial Response with 74% Tumor Reduction Patient Continues on Treatment Confirmed PR after 4 cycles Baseline 3/16/20 06/29/20 57-year-old woman with platinum resistant ovarian cancer progressing after 4 prior regimens received STRO-002 at 5.2 mg/kg and remains on study treatment
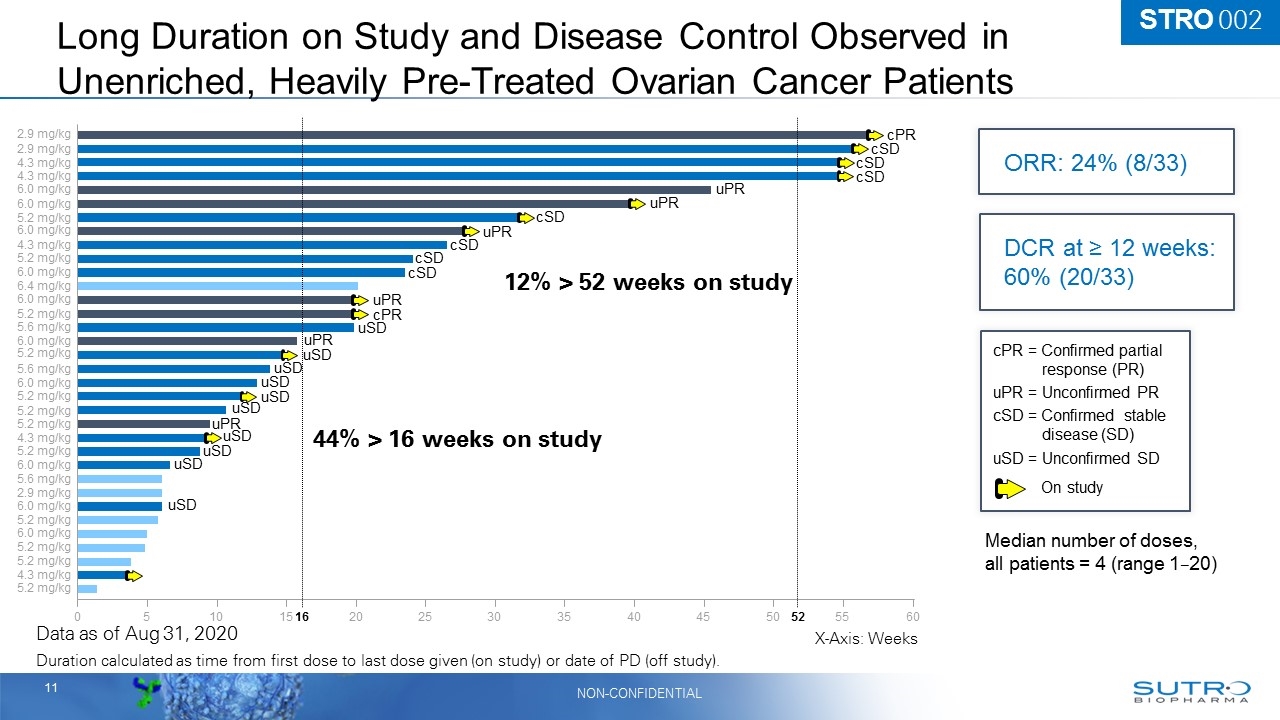
Long Duration on Study and Disease Control Observed in Unenriched, Heavily Pre-Treated Ovarian Cancer Patients Duration calculated as time from first dose to last dose given (on study) or date of PD (off study). Data as of Aug 31, 2020 STRO 002 0 5 10 15 20 25 30 35 40 45 50 55 60 2.9 mg/kg 2.9 mg/kg 6.0 mg/kg 5.6 mg/kg 4.3 mg/kg 4.3 mg/kg 6.0 mg/kg 5.2 mg/kg 6.0 mg/kg 4.3 mg/kg 5.2 mg/kg 6.0 mg/kg 6.0 mg/kg 5.2 mg/kg 6.0 mg/kg 5.2 mg/kg 5.6 mg/kg 6.0 mg/kg 5.2 mg/kg 5.2 mg/kg 5.2 mg/kg 4.3 mg/kg 5.2 mg/kg 6.0 mg/kg 6.0 mg/kg 6.4 mg/kg 5.6 mg/kg 2.9 mg/kg 5.2 mg/kg 6.0 mg/kg 5.2 mg/kg 5.2 mg/kg 4.3 mg/kg 5.2 mg/kg 16 52 On study Median number of doses, all patients = 4 (range 120) X-Axis: Weeks 44% > 16 weeks on study 12% > 52 weeks on study cPR = Confirmed partial response (PR) uPR = Unconfirmed PR cSD = Confirmed stable disease (SD) uSD = Unconfirmed SD cPR uPR uPR uPR uPR cPR uPR cSD cSD cSD cSD cSD cSD cSD uSD uSD uSD uSD uSD uSD uSD uSD uPR uSD uSD ORR: 24% (8/33) DCR at ≥ 12 weeks: 60% (20/33)
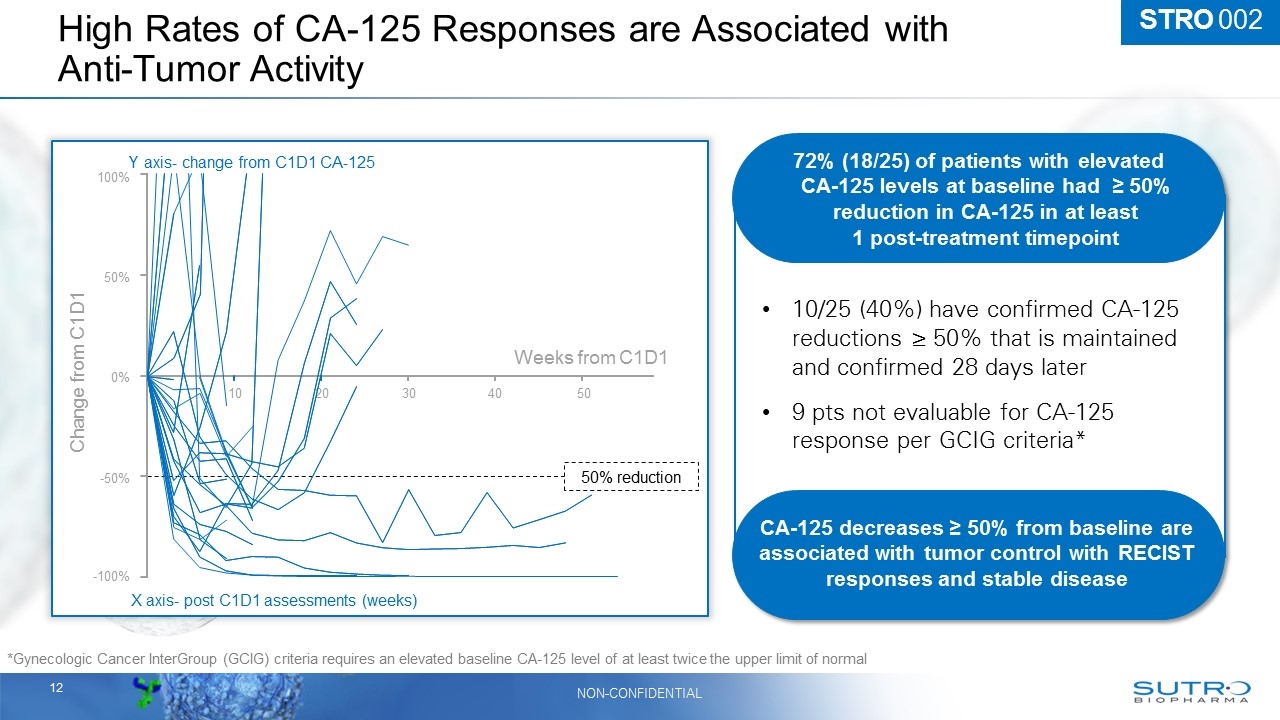
STRO 002 High Rates of CA-125 Responses are Associated with Anti-Tumor Activity *Gynecologic Cancer InterGroup (GCIG) criteria requires an elevated baseline CA-125 level of at least twice the upper limit of normal 10/25 (40%) have confirmed CA-125 reductions ≥ 50% that is maintained and confirmed 28 days later 9 pts not evaluable for CA-125 response per GCIG criteria* 72% (18/25) of patients with elevated CA-125 levels at baseline had ≥ 50% reduction in CA-125 in at least 1 post-treatment timepoint CA-125 decreases ≥ 50% from baseline are associated with tumor control with RECIST responses and stable disease X axis- post C1D1 assessments (weeks) Y axis- change from C1D1 CA-125 Weeks from C1D1 50% reduction Change from C1D1 100% 50% 0% -50% -100% 10 20 30 40 50
Promising Efficacy in a Heavily Pre-treated, Resistant/Refractory Unenriched Patient Population Improved efficacy outcomes (increased ORR and DCR) observed as data matures with longer follow-up Overall response rate is 24% (8/33) High DCR of 60% at ≥ 12 weeks and 47% at ≥ 16 weeks Population not enriched for high FRα expression 38% (13/34) of patients still on study with potential to further improve efficacy outcomes Heterogeneity of tumor regression and response, with some delayed partial responses observed after initial and variable period of stable disease High rate of CA-125 responses (≥ 50%) are associated with anti-tumor activity Data as of Aug 31, 2020

Acknowledgements Our gratitude to the women who chose to participate in this study and their families Thank you to the STRO-002 - GM1 investigators and study staff for their diligence in caring for these patients

Summary and Next Steps Arturo Molina, MD, MS
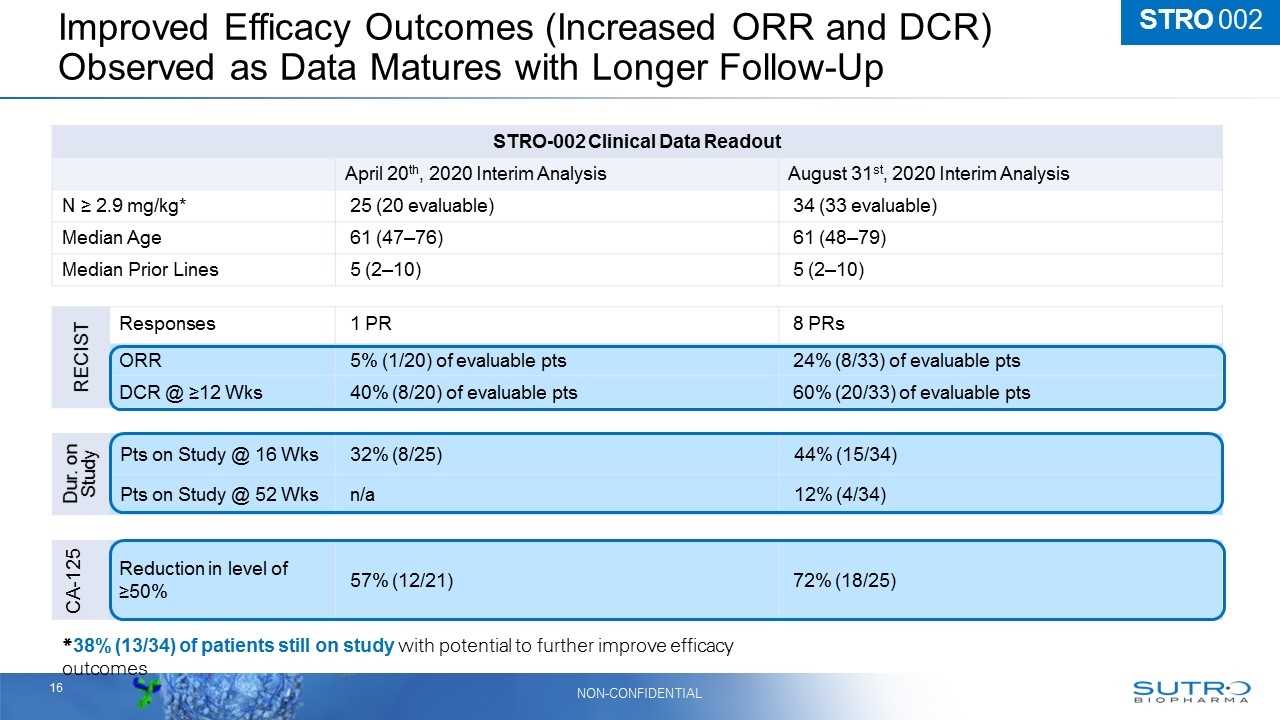
CA-125 Reduction in level of ≥50% 57% (12/21) 72% (18/25) STRO-002 Clinical Data Readout April 20th, 2020 Interim Analysis August 31st, 2020 Interim Analysis N ≥ 2.9 mg/kg* 25 (20 evaluable) 34 (33 evaluable) Median Age 61 (47–76) 61 (48–79) Median Prior Lines 5 (2–10) 5 (2–10) Improved Efficacy Outcomes (Increased ORR and DCR) Observed as Data Matures with Longer Follow-Up STRO 002 RECIST Responses 1 PR 8 PRs ORR 5% (1/20) of evaluable pts 24% (8/33) of evaluable pts DCR @ ≥12 Wks 40% (8/20) of evaluable pts 60% (20/33) of evaluable pts Dur. on Study Pts on Study @ 16 Wks 32% (8/25) 44% (15/34) Pts on Study @ 52 Wks n/a 12% (4/34) *38% (13/34) of patients still on study with potential to further improve efficacy outcomes

STRO 002 Summary and Next Steps in Clinical Development STRO-002 is clinically efficacious at multiple doses, starting at 2.9 mg/kg Dose reductions or delays were not associated with loss of anti-tumor activity Further dose optimization will be explored during dose expansion Anticipate RP2D will be in 4.3 – 5.2 mg/kg range Expansion cohort will seek to enroll less heavily pre-treated patients Monotherapy unenriched expansion cohort in ovarian cancer planned for 4Q20 Two FRα IHC assays are being compared for accuracy and consistency EOP1/2 FDA meeting planned for next year

FolRα – Targeting ADC Phase 1 Potential Best-in-Class ADC for Ovarian and Endometrial Cancers STRO 002