EX-99.1
Published on June 15, 2020

Company Overview June 2020 NASDAQ: STRO Bill Newell, CEO Exhibit 99.1

Forward Looking Statements This presentation and the accompanying oral presentation contain “forward-looking” statements that are based on our management’s beliefs and assumptions and on information currently available to management. Forward-looking statements include all statements other than statements of historical fact contained in this presentation, including information concerning our future financial performance, business plans and objectives, current and future clinical and preclinical activities, timing and success of our ongoing and planned clinical trials and related data, the timing of announcements, updates and results of our clinical trials and related data, timing and success of our planned development activities, our ability to obtain and maintain regulatory approval, the potential therapeutic benefits and economic value of our product candidates, potential growth opportunities, financing plans, competitive position, industry environment and potential market opportunities. Forward-looking statements are subject to known and unknown risks, uncertainties, assumptions and other factors, including risks and uncertainties related to our cash forecasts, our and our collaborators’ ability to advance our product candidates, the receipt and timing of potential regulatory submissions, designations, approvals and commercialization of product candidates, the timing and results of preclinical and clinical trials, and the expected impact of the COVID-19 pandemic on our operations. It is not possible for our management to predict all risks, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements we may make. These factors, together with those that may be described in greater detail under the heading “Risk Factors” contained in our most recent Annual Report on Form 10-K, Quarterly Report on Form 10-Q and other reports the company files from time to time with the Securities and Exchange Commission, may cause our actual results, performance or achievements to differ materially and adversely from those anticipated or implied by our forward-looking statements. You should not rely upon forward-looking statements as predictions of future events. Although our management believes that the expectations reflected in our forward-looking statements are reasonable, we cannot guarantee that the future results, levels of activity, performance or events and circumstances described in the forward-looking statements will be achieved or occur. Moreover, neither we nor our management assume responsibility for the accuracy and completeness of the forward-looking statements. We undertake no obligation to publicly update any forward-looking statements for any reason after the date of this presentation to conform these statements to actual results or to changes in our expectations, except as required by law. This presentation also contains estimates and other statistical data made by independent parties and by us relating to market size and growth and other data about our industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. In addition, projections, assumptions, and estimates of our future performance and the future performance of the markets in which we operate are necessarily subject to a high degree of uncertainty and risk. This presentation shall not constitute an offer to sell or the solicitation of an offer to buy, nor shall there be any sale of these securities in any state or other jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such state or other jurisdiction.

The Sutro Vision Changing the Future of Oncology PLATFORM FINANCIAL RESOURCES PARTNERS PEOPLE PRODUCTS 3 Clinical Programs in 2018-19 2 more projected in 2020-21 Team With Proven Track Record of Executing Cash, cash equivalents, and marketable securities balance of $129.6M as of March 31, 2020 Plus gross proceeds of ~$98M from follow-on financing completed May 2020 Estimated cash runway into 2022 (including financing net proceeds) XpressCF® Precision Protein Engineering Generates Superior Molecules Integrated Manufacturing Facility Is Strategic Premier Collaborations: Bristol Myers Squibb – BCMA ADC in Ph 1 Merck – Promising Cytokine DDerivatives EMD Serono – MUC1-EGFR Bispecific T ADC at GMP Supply iiiiStage. AACR virtual iiiipresentation on iiiiJune 24th.
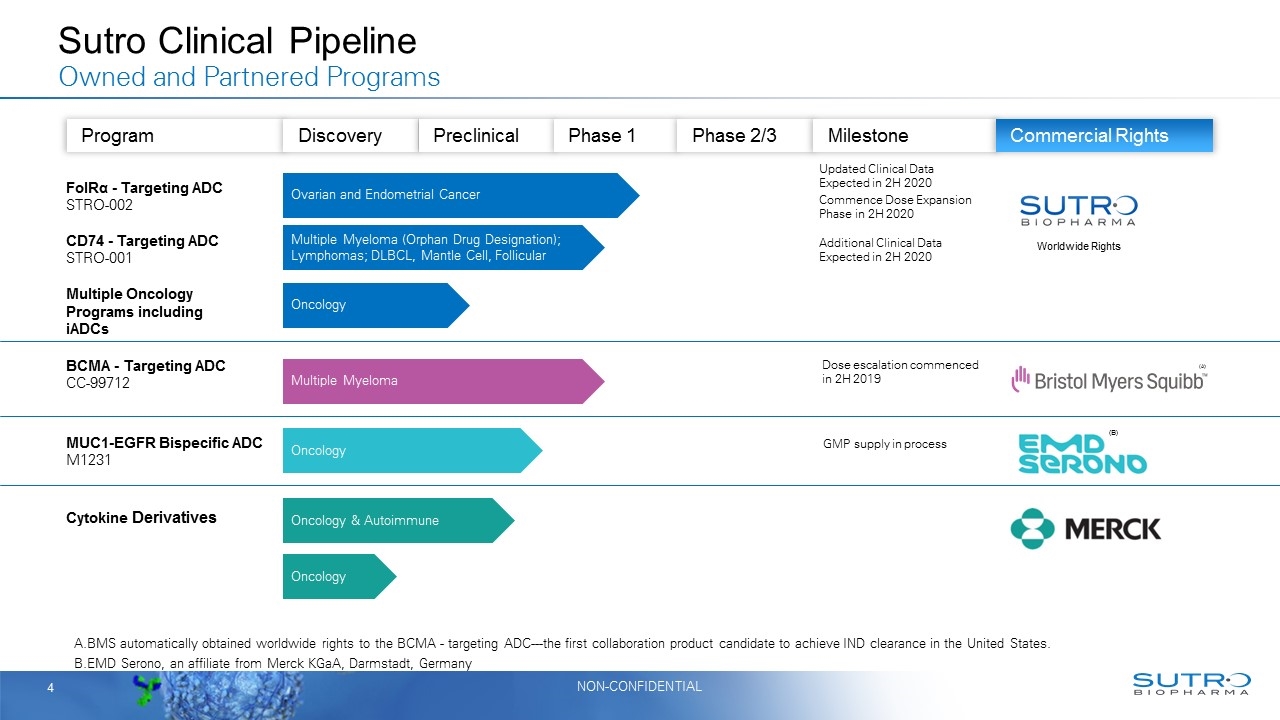
Sutro Clinical Pipeline Owned and Partnered Programs Program Discovery Preclinical Phase 1 Phase 2/3 Milestone Commercial Rights Ovarian and Endometrial Cancer FolRα - Targeting ADC STRO-002 CD74 - Targeting ADC STRO-001 Multiple Oncology Programs including iADCs Cytokine Derivatives Commence Dose Expansion Phase in 2H 2020 Updated Clinical Data Expected in 2H 2020 Dose escalation commenced in 2H 2019 GMP supply in process Multiple Myeloma (Orphan Drug Designation); Lymphomas; DLBCL, Mantle Cell, Follicular Oncology Multiple Myeloma Oncology Oncology & Autoimmune Oncology Worldwide Rights (A) (B) BCMA - Targeting ADC CC-99712 BMS automatically obtained worldwide rights to the BCMA - targeting ADC---the first collaboration product candidate to achieve IND clearance in the United States. EMD Serono, an affiliate from Merck KGaA, Darmstadt, Germany Additional Clinical Data Expected in 2H 2020 MUC1-EGFR Bispecific ADC M1231
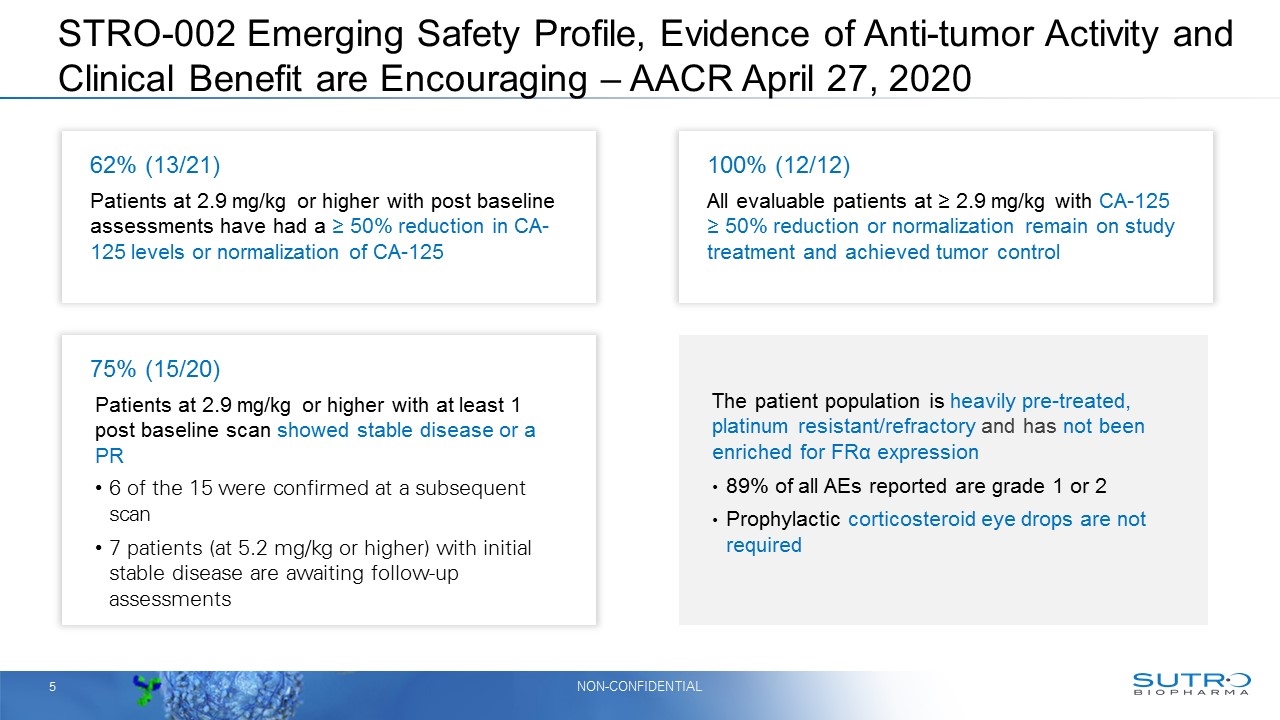
STRO-002 Emerging Safety Profile, Evidence of Anti-tumor Activity and Clinical Benefit are Encouraging – AACR April 27, 2020 62% (13/21) Patients at 2.9 mg/kg or higher with post baseline assessments have had a ≥ 50% reduction in CA-125 levels or normalization of CA-125 100% (12/12) All evaluable patients at ≥ 2.9 mg/kg with CA-125 ≥ 50% reduction or normalization remain on study treatment and achieved tumor control 75% (15/20) Patients at 2.9 mg/kg or higher with at least 1 post baseline scan showed stable disease or a PR 6 of the 15 were confirmed at a subsequent scan 7 patients (at 5.2 mg/kg or higher) with initial stable disease are awaiting follow-up assessments The patient population is heavily pre-treated, platinum resistant/refractory and has not been enriched for FRα expression 89% of all AEs reported are grade 1 or 2 Prophylactic corticosteroid eye drops are not required
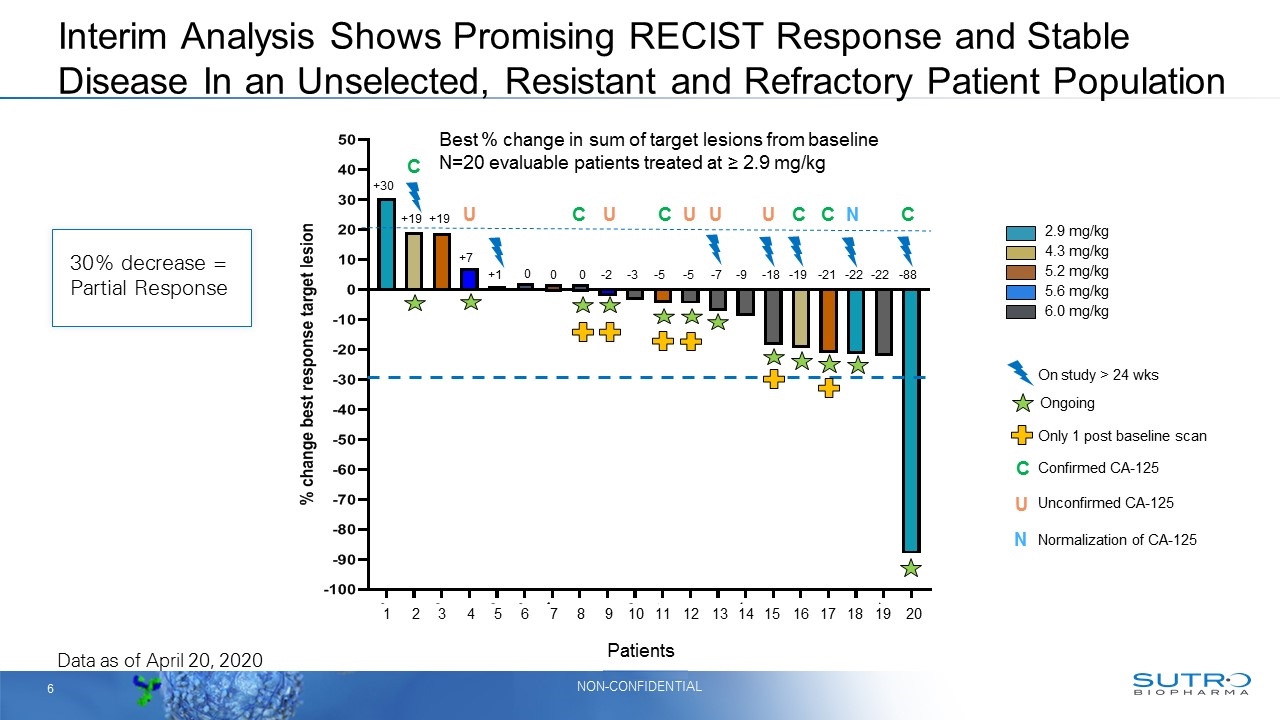
Interim Analysis Shows Promising RECIST Response and Stable Disease In an Unselected, Resistant and Refractory Patient Population 2.9 mg/kg 4.3 mg/kg 6.0 mg/kg 5.2 mg/kg 5.6 mg/kg Ongoing Best % change in sum of target lesions from baseline N=20 evaluable patients treated at ≥ 2.9 mg/kg +30 +19 +19 +7 +1 0 0 0 -2 -3 -5 -5 -7 -9 -18 -19 -21 -22 -22 -88 Only 1 post baseline scan On study > 24 wks Data as of April 20, 2020 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 C C C C C C U U U U U N C Confirmed CA-125 Unconfirmed CA-125 U N Normalization of CA-125 Patients 30% decrease = Partial Response

CD74-targeting ADC (STRO-001) Phase 1 initial safety and efficacy data in multiple myeloma (MM) and NHL 33 patients enrolled, heavily pretreated (Jan 2020) MTD not yet determined Dose escalation continuing above 0.91 mg/kg No ocular toxicity observed & generally well tolerated 1 CR and 1 PR observed in two patients with DLBCL; stable disease for one patient with MM (in first 25 MM/NHL patients) Orphan Drug Designation for MM BCMA-targeting ADC (CC-99712) Phase 1 trial for MM by BMS (formerly Celgene) Dose escalation phase commenced in 2H 2019 Two Additional Phase 1 Programs Progressing

Sutro’s Anticipated Upcoming Milestones: 2020 Milestones for Sutro Proprietary Programs Anticipated Timing FolRα-targeting ADC (STRO-002) - Phase 1 Additional dose escalation clinical data Updated dose escalation clinical data Commence dose expansion 2Q 2020 2H 2020 2H 2020 CD74-targeting ADC (STRO-001) - Phase 1 Additional dose escalation clinical data Commence dose expansion 2H 2020 1H 2021 STRO-003 IND candidate selected & IND timing projected 2H 2020 Additional potential milestones in 2020: BMS: Phase 1 clinical development update for CC-99712 Merck: Progress on first cytokine derivative program EMD Serono:Plans for Phase 1 clinical development of MUC1-EGFR Bispecific ADC
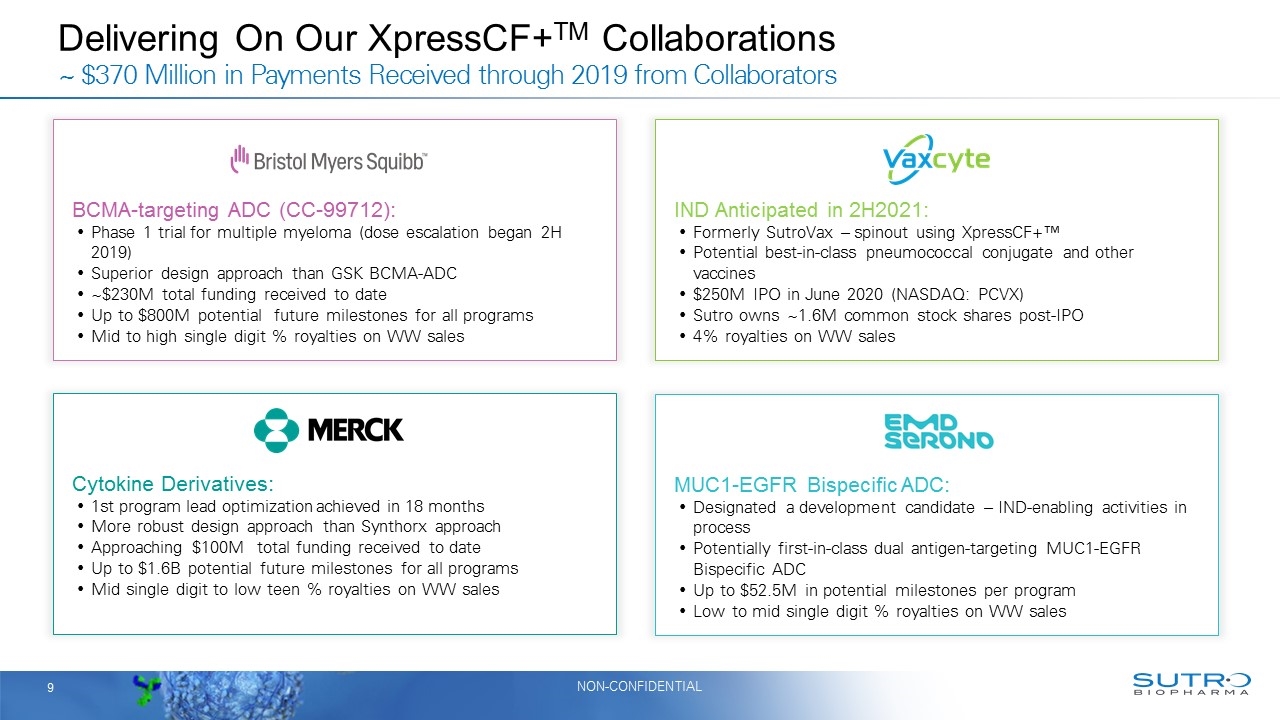
Delivering On Our XpressCF+TM Collaborations ~ $370 Million in Payments Received through 2019 from Collaborators Cytokine Derivatives: 1st program lead optimization achieved in 18 months More robust design approach than Synthorx approach Approaching $100M total funding received to date Up to $1.6B potential future milestones for all programs Mid single digit to low teen % royalties on WW sales BCMA-targeting ADC (CC-99712): Phase 1 trial for multiple myeloma (dose escalation began 2H 2019) Superior design approach than GSK BCMA-ADC ~$230M total funding received to date Up to $800M potential future milestones for all programs Mid to high single digit % royalties on WW sales MUC1-EGFR Bispecific ADC: Designated a development candidate – IND-enabling activities in process Potentially first-in-class dual antigen-targeting MUC1-EGFR Bispecific ADC Up to $52.5M in potential milestones per program Low to mid single digit % royalties on WW sales IND Anticipated in 2H2021: Formerly SutroVax – spinout using XpressCF+™ Potential best-in-class pneumococcal conjugate and other vaccines $250M IPO in June 2020 (NASDAQ: PCVX) Sutro owns ~1.6M common stock shares post-IPO 4% royalties on WW sales
10 Sutro’s Approach Delivering Potentially Best-in-Class ADCs and Cytokine Conjugates Widening the Therapeutic Index is Key to Achieving Best-in-Class Performance The Sutro Advantage Rapid iterative design to identify best-in-class product candidates Selection of specific sites for conjugation for optimal performance Homogenous end-products Cytokine Receptor Targets Rapid evolution of optimal attributes to enable systemic administration ADCs, iADCs & Targeted Therapeutics Precision delivery of active pharmacological entity with optimal attributes is key XpressCF® Our Truly Empirical Approach Proprietary XpressCF® rapid synthesis protein library generation, precision conjugation technology and robust medicinal chemistry to enable: Optimization of known product concepts Empirical evaluation of unexplored product concepts Rapid generation of best-in-class molecules

FolRα – Targeting ADC Phase 1 Potential Best-in-Class ADC for Ovarian and Endometrial Cancers STRO 002
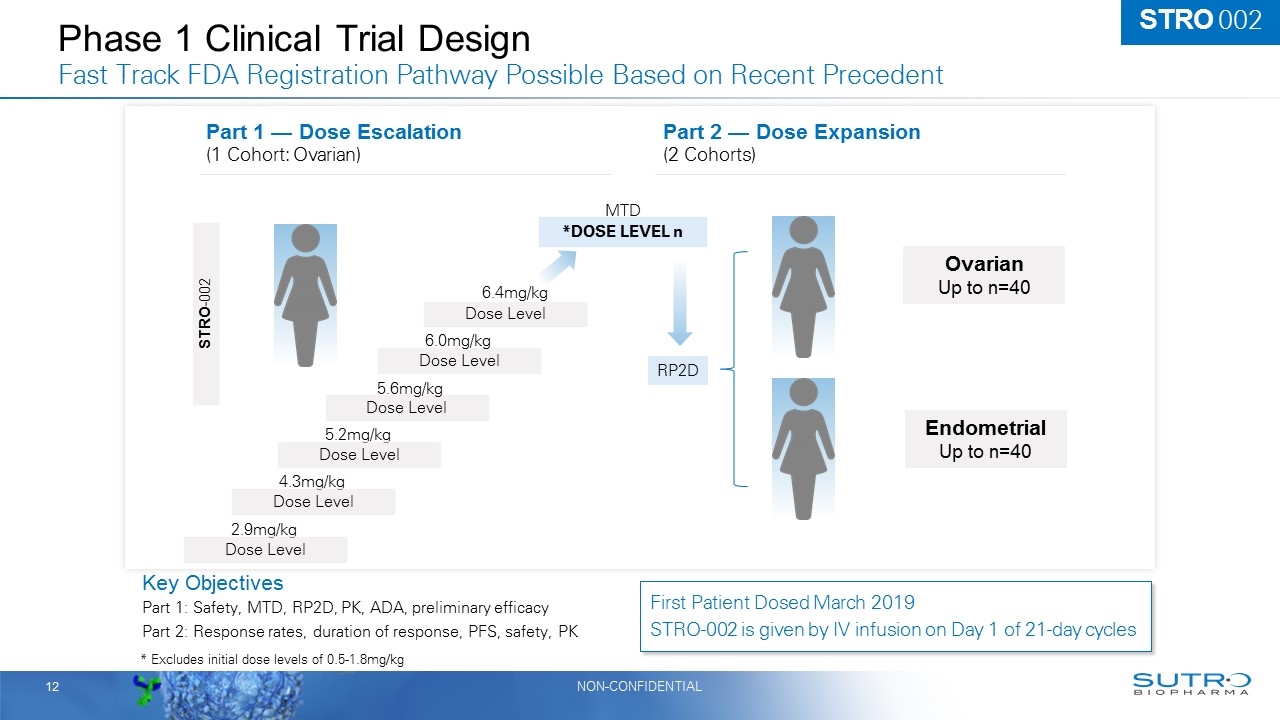
Phase 1 Clinical Trial Design Fast Track FDA Registration Pathway Possible Based on Recent Precedent Key Objectives Part 1: Safety, MTD, RP2D, PK, ADA, preliminary efficacy Part 2: Response rates, duration of response, PFS, safety, PK *DOSE LEVEL n Part 2 — Dose Expansion (2 Cohorts) STRO-002 Dose Level Dose Level Dose Level 5.2mg/kg 5.6mg/kg 6.4mg/kg Part 1 — Dose Escalation (1 Cohort: Ovarian) MTD RP2D Endometrial Up to n=40 Ovarian Up to n=40 STRO 002 First Patient Dosed March 2019 STRO-002 is given by IV infusion on Day 1 of 21-day cycles 12 * Excludes initial dose levels of 0.5-1.8mg/kg Dose Level 6.0mg/kg 4.3mg/kg Dose Level 2.9mg/kg Dose Level
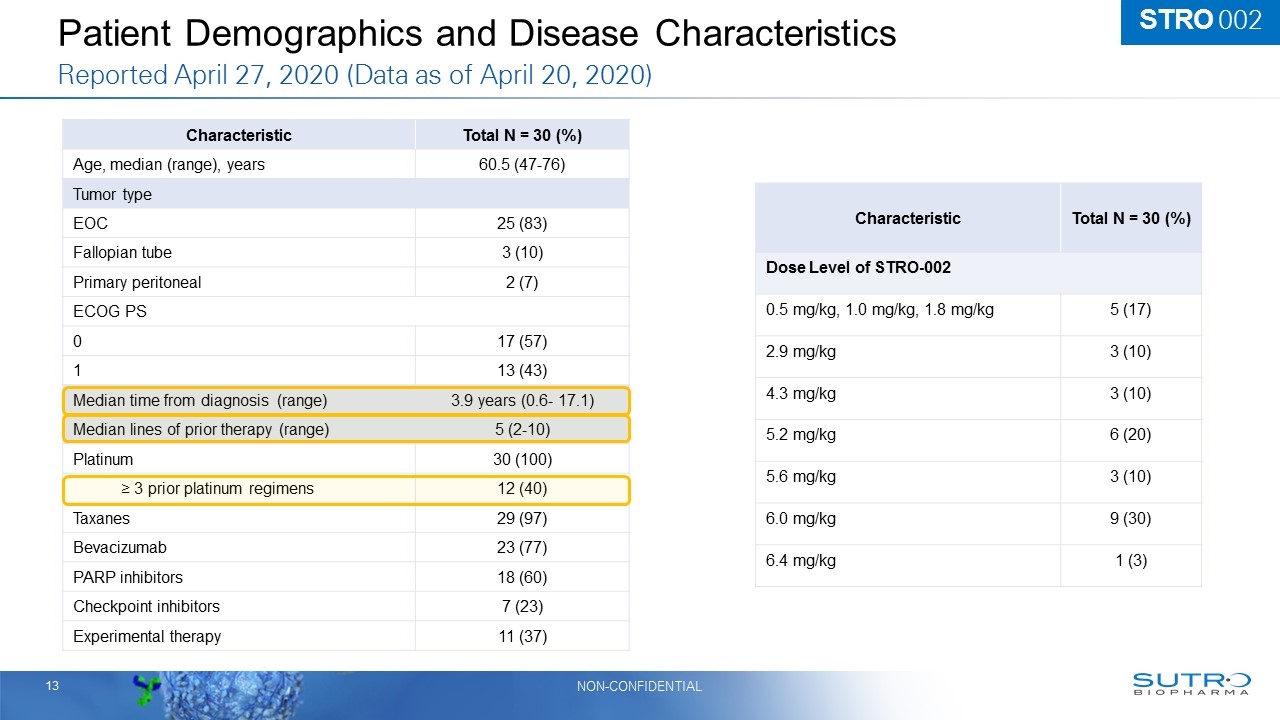
Patient Demographics and Disease Characteristics Reported April 27, 2020 (Data as of April 20, 2020) Characteristic Total N = 30 (%) Age, median (range), years 60.5 (47-76) Tumor type EOC 25 (83) Fallopian tube 3 (10) Primary peritoneal 2 (7) ECOG PS 0 17 (57) 1 13 (43) Median time from diagnosis (range) 3.9 years (0.6- 17.1) Median lines of prior therapy (range) 5 (2-10) Platinum 30 (100) ≥ 3 prior platinum regimens 12 (40) Taxanes 29 (97) Bevacizumab 23 (77) PARP inhibitors 18 (60) Checkpoint inhibitors 7 (23) Experimental therapy 11 (37) Characteristic Total N = 30 (%) Dose Level of STRO-002 0.5 mg/kg, 1.0 mg/kg, 1.8 mg/kg 5 (17) 2.9 mg/kg 3 (10) 4.3 mg/kg 3 (10) 5.2 mg/kg 6 (20) 5.6 mg/kg 3 (10) 6.0 mg/kg 9 (30) 6.4 mg/kg 1 (3) STRO 002
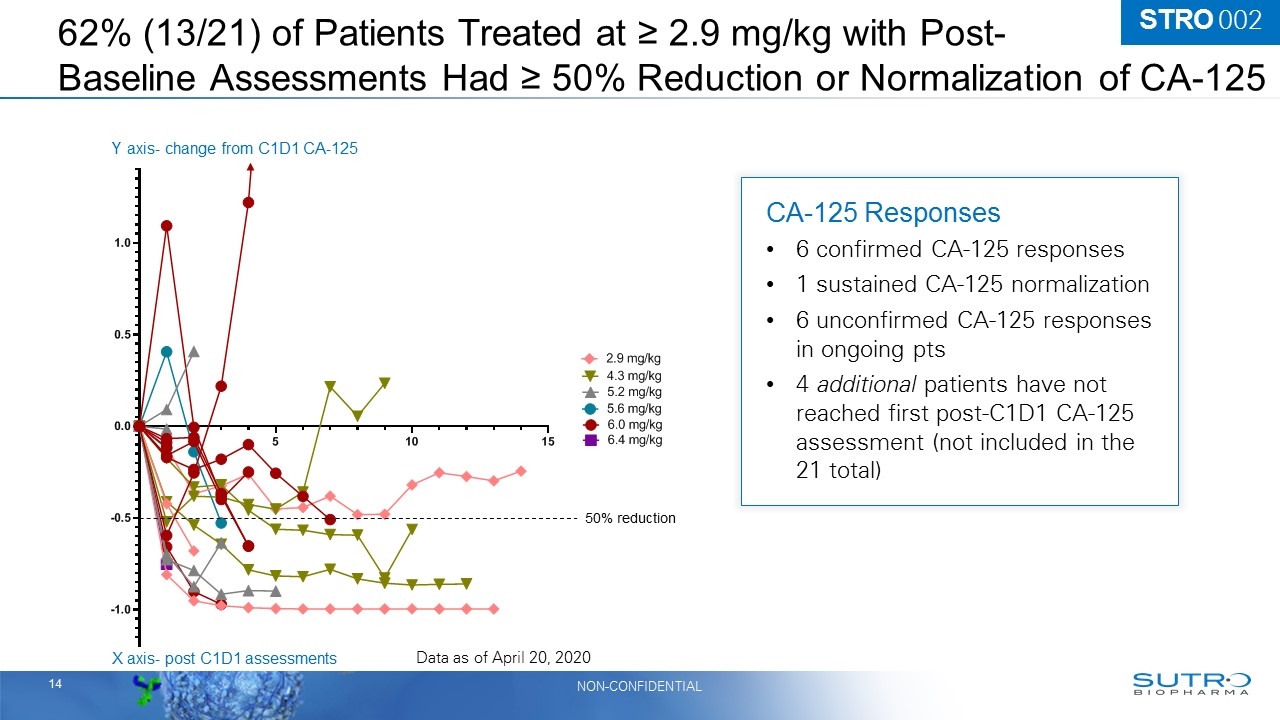
62% (13/21) of Patients Treated at ≥ 2.9 mg/kg with Post- Baseline Assessments Had ≥ 50% Reduction or Normalization of CA-125 50% reduction X axis- post C1D1 assessments Y axis- change from C1D1 CA-125 Data as of April 20, 2020 CA-125 Responses 6 confirmed CA-125 responses 1 sustained CA-125 normalization 6 unconfirmed CA-125 responses in ongoing pts 4 additional patients have not reached first post-C1D1 CA-125 assessment (not included in the 21 total) STRO 002
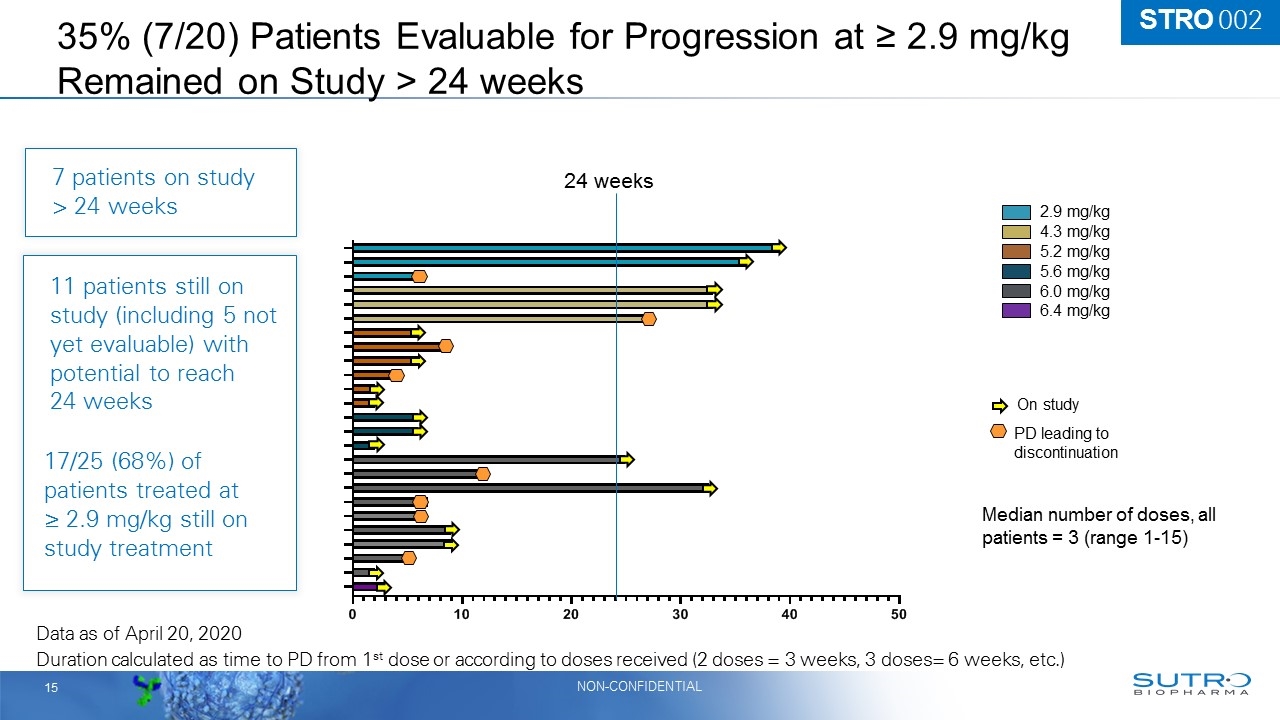
35% (7/20) Patients Evaluable for Progression at ≥ 2.9 mg/kg Remained on Study > 24 weeks 2.9 mg/kg 4.3 mg/kg 6.0 mg/kg Duration calculated as time to PD from 1st dose or according to doses received (2 doses = 3 weeks, 3 doses= 6 weeks, etc.) 5.2 mg/kg 5.6 mg/kg 6.4 mg/kg On study PD leading to discontinuation Median number of doses, all patients = 3 (range 1-15) Data as of April 20, 2020 11 patients still on study (including 5 not yet evaluable) with potential to reach 24 weeks 17/25 (68%) of patients treated at ≥ 2.9 mg/kg still on study treatment 24 weeks 7 patients on study > 24 weeks STRO 002
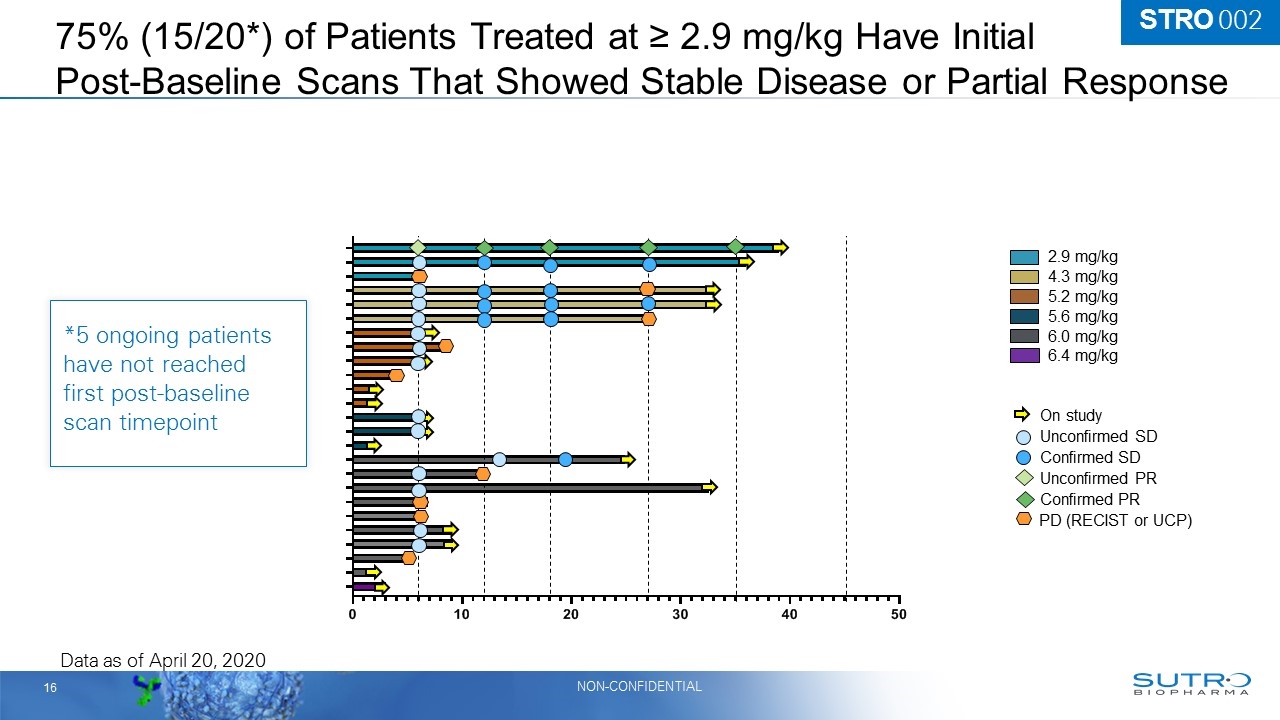
75% (15/20*) of Patients Treated at ≥ 2.9 mg/kg Have Initial Post-Baseline Scans That Showed Stable Disease or Partial Response 2.9 mg/kg 4.3 mg/kg 6.0 mg/kg 5.2 mg/kg 5.6 mg/kg 6.4 mg/kg On study Confirmed PR Unconfirmed SD Confirmed SD Unconfirmed PR PD (RECIST or UCP) Data as of April 20, 2020 On study On study On study On study On study On study On study On study On study On study On study On study On study On study On study On study On study *5 ongoing patients have not reached first post-baseline scan timepoint STRO 002
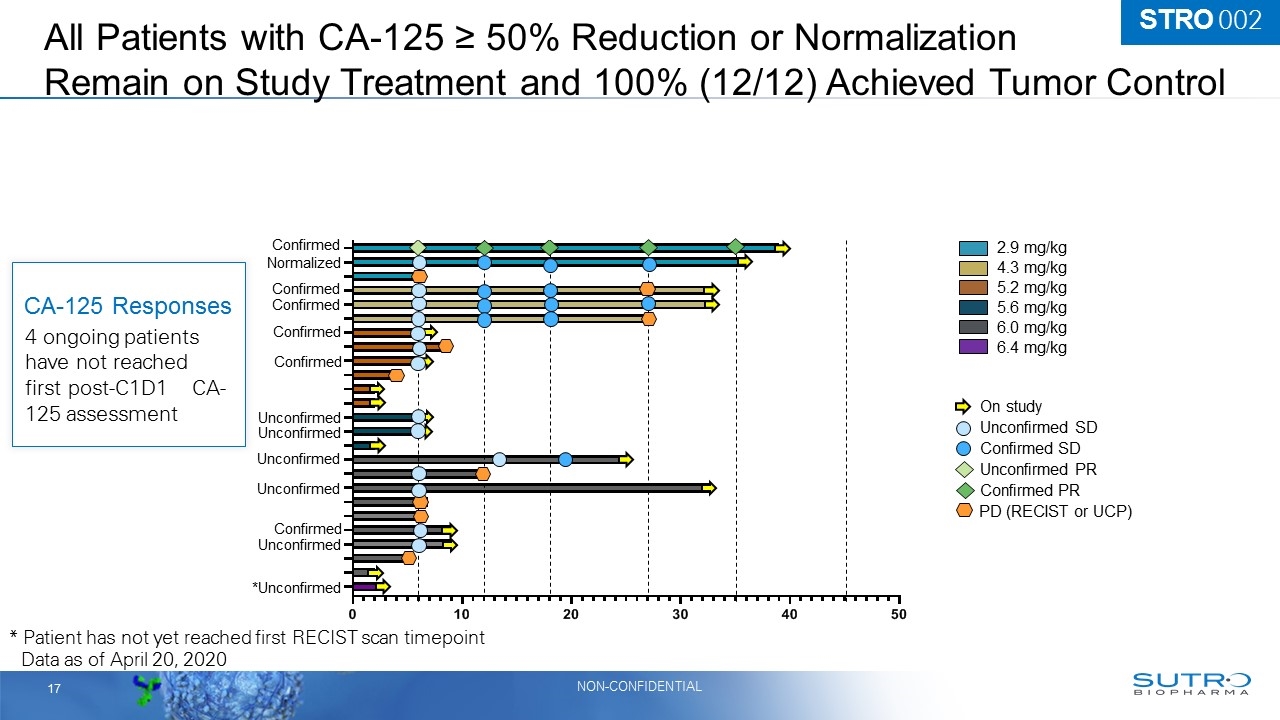
All Patients with CA-125 ≥ 50% Reduction or Normalization Remain on Study Treatment and 100% (12/12) Achieved Tumor Control 2.9 mg/kg 4.3 mg/kg 6.0 mg/kg 5.2 mg/kg 5.6 mg/kg 6.4 mg/kg On study Confirmed PR Unconfirmed SD Confirmed SD Unconfirmed PR PD (RECIST or UCP) Data as of April 20, 2020 Confirmed Unconfirmed Unconfirmed Confirmed Confirmed Confirmed Confirmed Normalized Unconfirmed Unconfirmed *Unconfirmed Unconfirmed 4 ongoing patients have not reached first post-C1D1 CA-125 assessment CA-125 Responses Confirmed * Patient has not yet reached first RECIST scan timepoint STRO 002
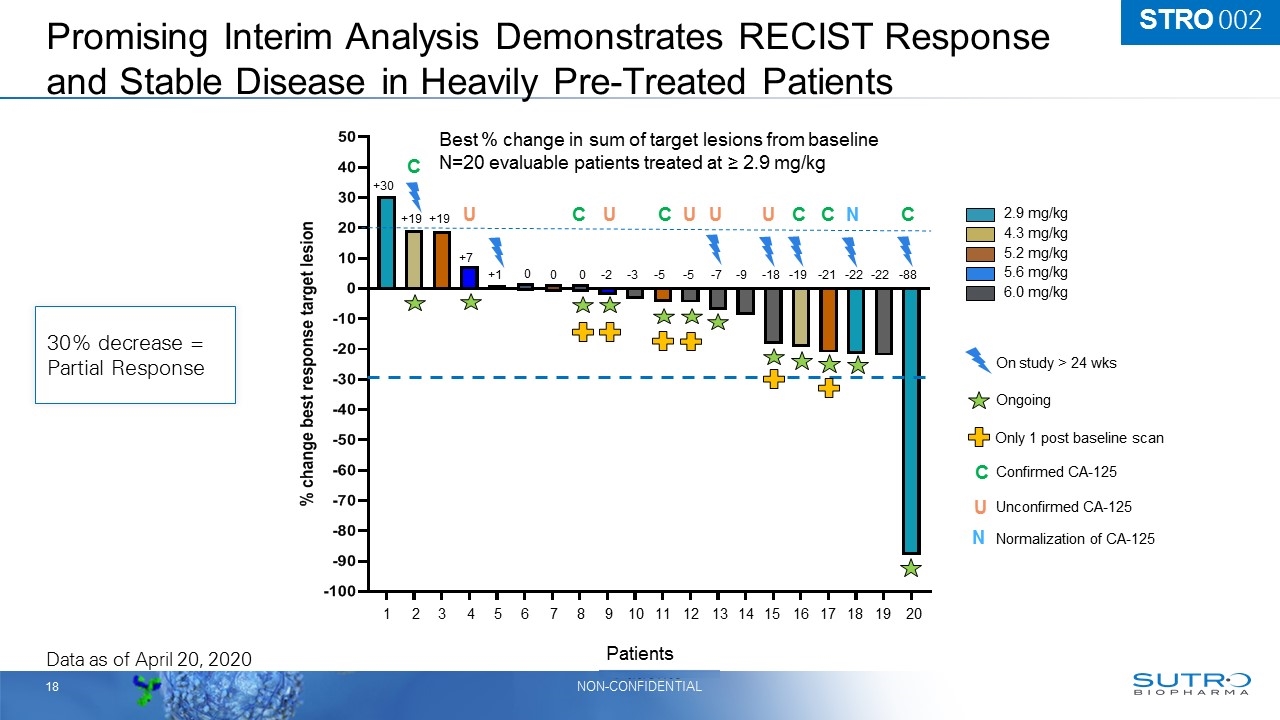
2.9 mg/kg 4.3 mg/kg 6.0 mg/kg 5.2 mg/kg 5.6 mg/kg Ongoing Best % change in sum of target lesions from baseline N=20 evaluable patients treated at ≥ 2.9 mg/kg +30 +19 +19 +7 +1 0 0 0 -2 -3 -5 -5 -7 -9 -18 -19 -21 -22 -22 -88 Only 1 post baseline scan On study > 24 wks Data as of April 20, 2020 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 C C C C C C U U U U U N C Confirmed CA-125 Unconfirmed CA-125 U N Normalization of CA-125 Promising Interim Analysis Demonstrates RECIST Response and Stable Disease in Heavily Pre-Treated Patients 30% decrease = Partial Response STRO 002 Patients
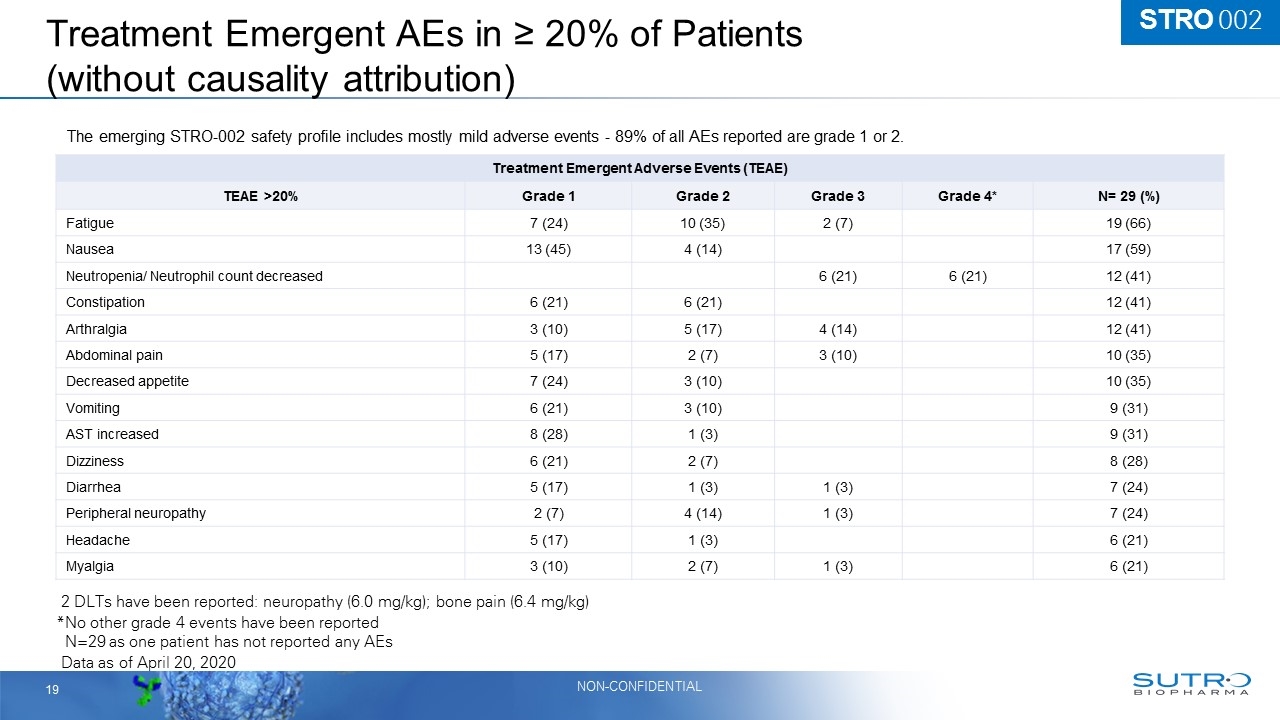
Treatment Emergent AEs in ≥ 20% of Patients (without causality attribution) Data as of April 20, 2020 Treatment Emergent Adverse Events (TEAE) TEAE >20% Grade 1 Grade 2 Grade 3 Grade 4* N= 29 (%) Fatigue 7 (24) 10 (35) 2 (7) 19 (66) Nausea 13 (45) 4 (14) 17 (59) Neutropenia/ Neutrophil count decreased 6 (21) 6 (21) 12 (41) Constipation 6 (21) 6 (21) 12 (41) Arthralgia 3 (10) 5 (17) 4 (14) 12 (41) Abdominal pain 5 (17) 2 (7) 3 (10) 10 (35) Decreased appetite 7 (24) 3 (10) 10 (35) Vomiting 6 (21) 3 (10) 9 (31) AST increased 8 (28) 1 (3) 9 (31) Dizziness 6 (21) 2 (7) 8 (28) Diarrhea 5 (17) 1 (3) 1 (3) 7 (24) Peripheral neuropathy 2 (7) 4 (14) 1 (3) 7 (24) Headache 5 (17) 1 (3) 6 (21) Myalgia 3 (10) 2 (7) 1 (3) 6 (21) The emerging STRO-002 safety profile includes mostly mild adverse events - 89% of all AEs reported are grade 1 or 2. *No other grade 4 events have been reported *N=29 as one patient has not reported any AEs STRO 002 2 DLTs have been reported: neuropathy (6.0 mg/kg); bone pain (6.4 mg/kg)

Summary - Well Tolerated, Encouraging Clinical Benefit Expansion Cohorts Planned for 2H 2020 STRO-002 was generally well tolerated and mostly associated with mild events 5/30= 17% have not had post-treatment scan for initial RECIST assessment 89% of all AEs reported are grade 1 or 2 Prophylactic corticosteroid eye drops have not been required MTD has not been reached, additional patients are being enrolled in the 5.2mg/kg – 6.0mg/kg range to better characterize RP2D Follow-up is still early and enrollment ongoing 5/30 = 17% have not had post-treatment scan for initial RECIST assessment Next Steps: Recommended Phase 2 dose to be confirmed Expansion cohorts planned for 2H 2020 STRO 002
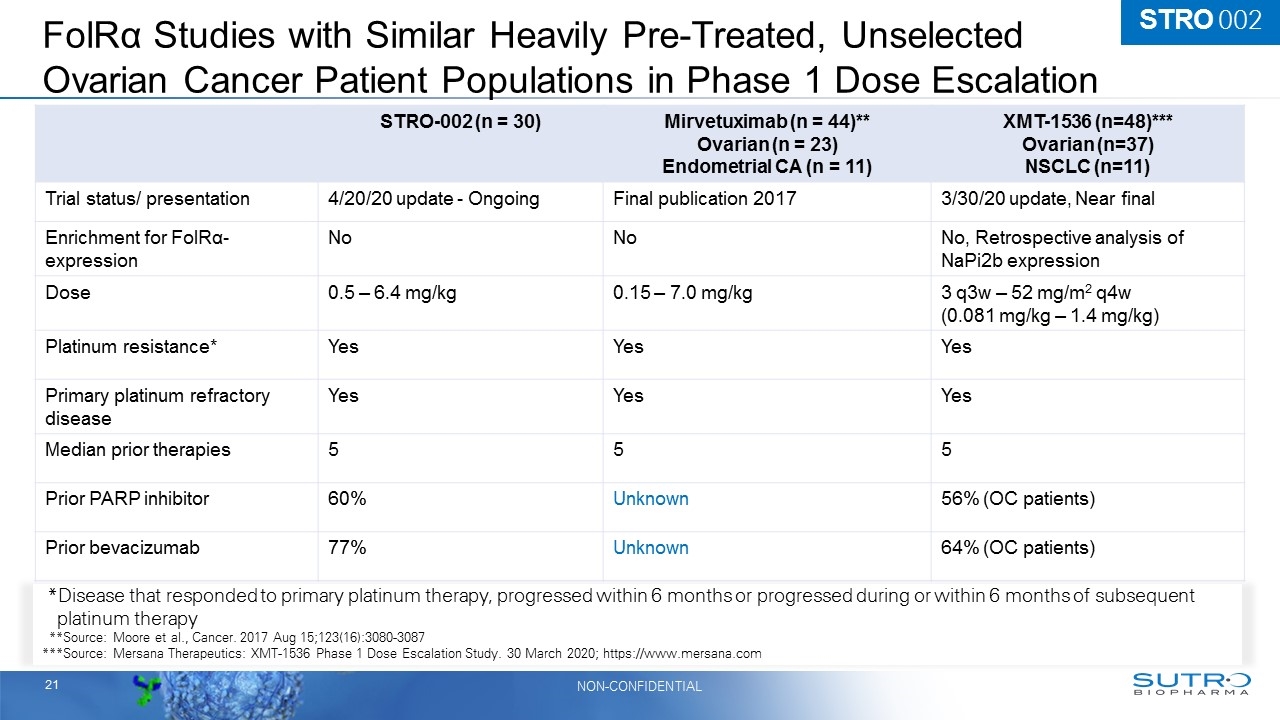
FolRα Studies with Similar Heavily Pre-Treated, Unselected Ovarian Cancer Patient Populations in Phase 1 Dose Escalation STRO-002 (n = 30) Mirvetuximab (n = 44)** Ovarian (n = 23) Endometrial CA (n = 11) XMT-1536 (n=48)*** Ovarian (n=37) NSCLC (n=11) Trial status/ presentation 4/20/20 update - Ongoing Final publication 2017 3/30/20 update, Near final Enrichment for FolRα-expression No No No, Retrospective analysis of NaPi2b expression Dose 0.5 – 6.4 mg/kg 0.15 – 7.0 mg/kg 3 q3w – 52 mg/m2 q4w (0.081 mg/kg – 1.4 mg/kg) Platinum resistance* Yes Yes Yes Primary platinum refractory disease Yes Yes Yes Median prior therapies 5 5 5 Prior PARP inhibitor 60% Unknown 56% (OC patients) Prior bevacizumab 77% Unknown 64% (OC patients) *Disease that responded to primary platinum therapy, progressed within 6 months or progressed during or within 6 months of subsequent platinum therapy ***Source: Moore et al., Cancer. 2017 Aug 15;123(16):3080-3087 ***Source: Mersana Therapeutics: XMT-1536 Phase 1 Dose Escalation Study. 30 March 2020; https://www.mersana.com STRO 002
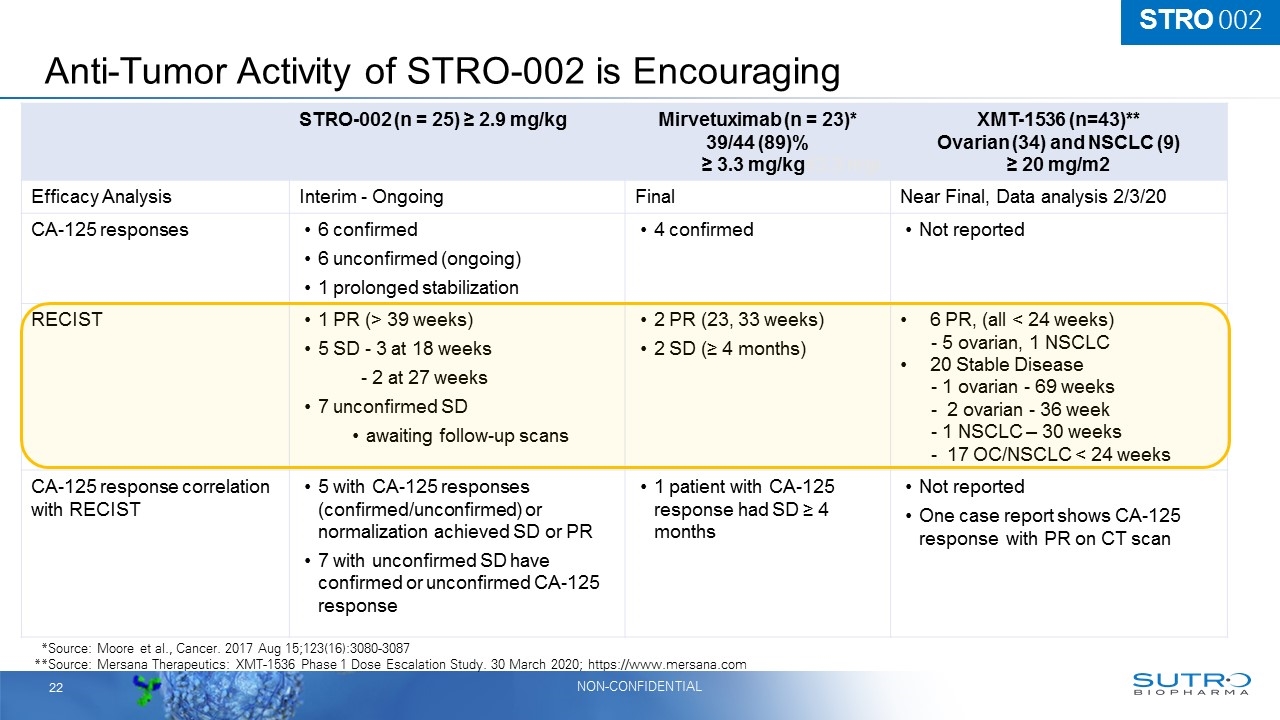
Anti-Tumor Activity of STRO-002 is Encouraging STRO-002 (n = 25) ≥ 2.9 mg/kg Mirvetuximab (n = 23)* 39/44 (89)% ≥ 3.3 mg/kg43.3 mg/ XMT-1536 (n=43)** Ovarian (34) and NSCLC (9) ≥ 20 mg/m2 Efficacy Analysis Interim - Ongoing Final Near Final, Data analysis 2/3/20 CA-125 responses 6 confirmed 6 unconfirmed (ongoing) 1 prolonged stabilization 4 confirmed Not reported RECIST 1 PR (> 39 weeks) 5 SD - 3 at 18 weeks - 2 at 27 weeks 7 unconfirmed SD awaiting follow-up scans 2 PR (23, 33 weeks) 2 SD (≥ 4 months) 6 PR, (all < 24 weeks) - 5 ovarian, 1 NSCLC 20 Stable Disease - 1 ovarian - 69 weeks - 2 ovarian - 36 week - 1 NSCLC – 30 weeks - 17 OC/NSCLC < 24 weeks CA-125 response correlation with RECIST 5 with CA-125 responses (confirmed/unconfirmed) or normalization achieved SD or PR 7 with unconfirmed SD have confirmed or unconfirmed CA-125 response 1 patient with CA-125 response had SD ≥ 4 months Not reported One case report shows CA-125 response with PR on CT scan **Source: Moore et al., Cancer. 2017 Aug 15;123(16):3080-3087 **Source: Mersana Therapeutics: XMT-1536 Phase 1 Dose Escalation Study. 30 March 2020; https://www.mersana.com STRO 002
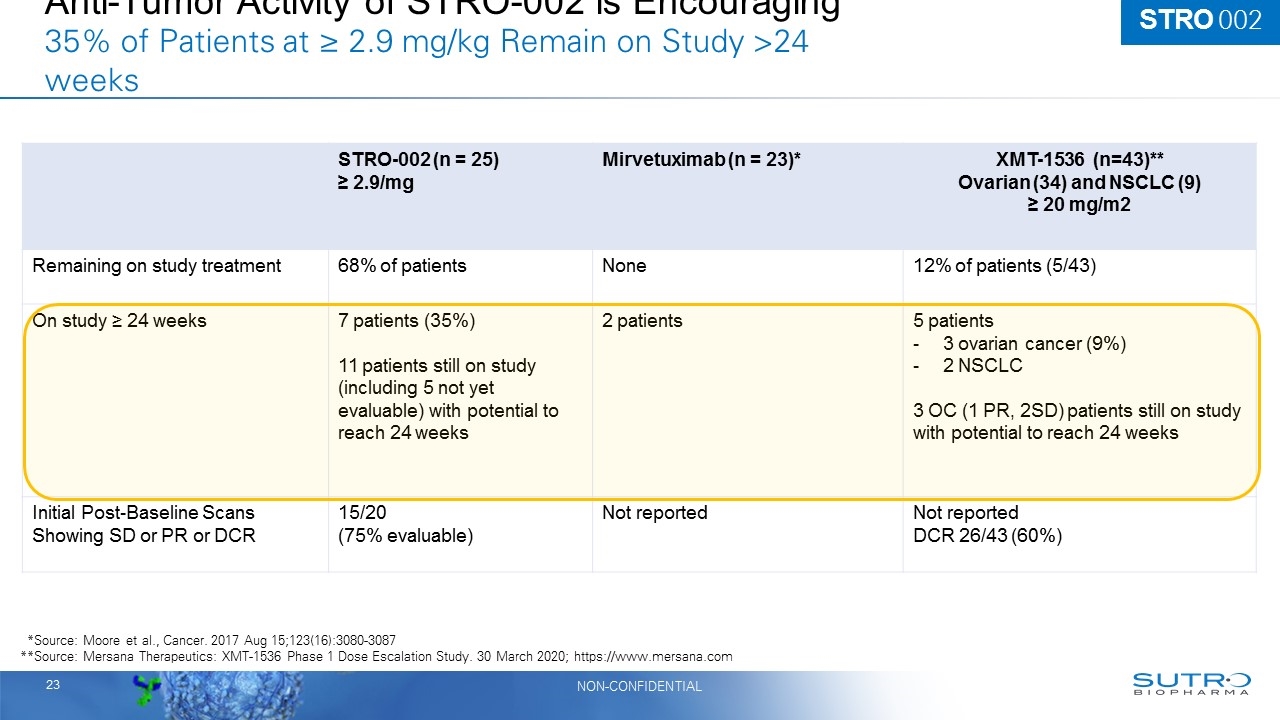
Anti-Tumor Activity of STRO-002 is Encouraging 35% of Patients at ≥ 2.9 mg/kg Remain on Study >24 weeks STRO-002 (n = 25) ≥ 2.9/mg Mirvetuximab (n = 23)* XMT-1536 (n=43)** Ovarian (34) and NSCLC (9) ≥ 20 mg/m2 Remaining on study treatment 68% of patients None 12% of patients (5/43) On study ≥ 24 weeks 7 patients (35%) 11 patients still on study (including 5 not yet evaluable) with potential to reach 24 weeks 2 patients 5 patients 3 ovarian cancer (9%) 2 NSCLC 3 OC (1 PR, 2SD) patients still on study with potential to reach 24 weeks Initial Post-Baseline Scans Showing SD or PR or DCR 15/20 (75% evaluable) Not reported Not reported DCR 26/43 (60%) **Source: Moore et al., Cancer. 2017 Aug 15;123(16):3080-3087 **Source: Mersana Therapeutics: XMT-1536 Phase 1 Dose Escalation Study. 30 March 2020; https://www.mersana.com STRO 002

CD74-Targeting ADC: Phase 1 Potential First and Best-in-Class ADC for B-Cell Malignancies STRO 001
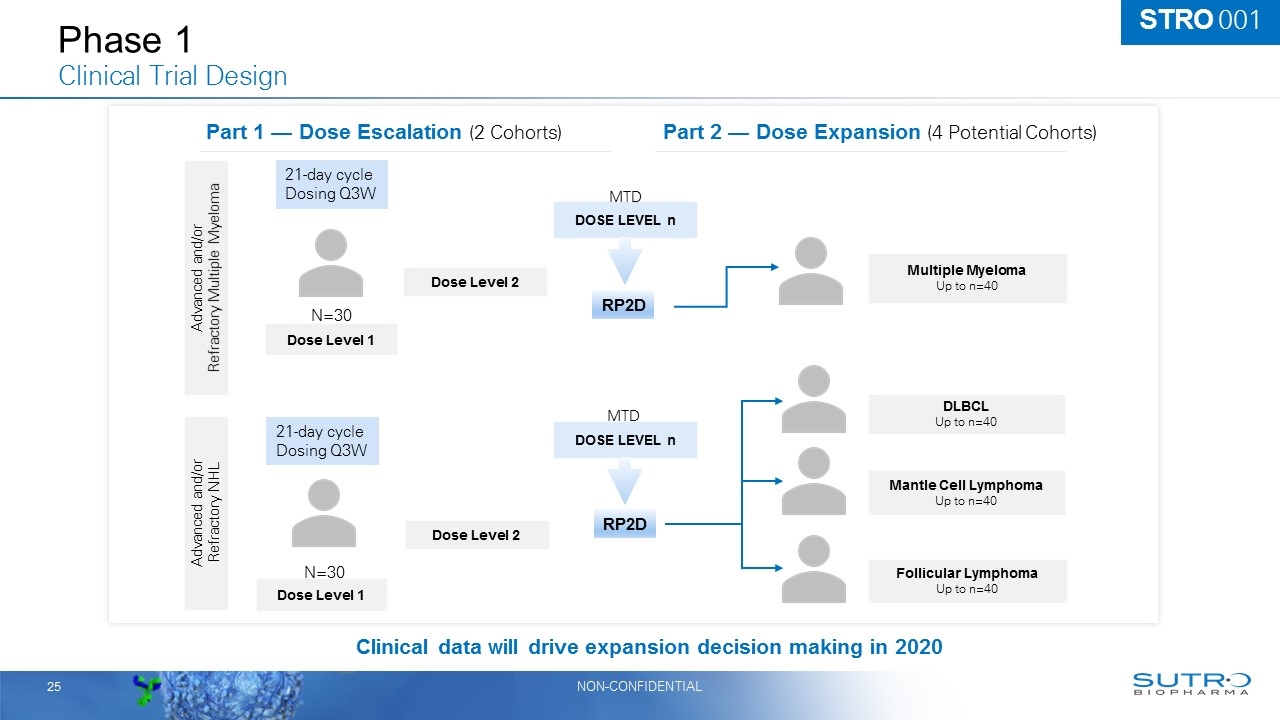
Phase 1 Clinical Trial Design Clinical data will drive expansion decision making in 2020 Part 2 — Dose Expansion (4 Potential Cohorts) Part 1 — Dose Escalation (2 Cohorts) Dose Level 1 Dose Level 2 DOSE LEVEL n Advanced and/or Refractory Multiple Myeloma Advanced and/or Refractory NHL Multiple Myeloma Up to n=40 DLBCL Up to n=40 Mantle Cell Lymphoma Up to n=40 Follicular Lymphoma Up to n=40 Dose Level 1 Dose Level 2 DOSE LEVEL n 21-day cycle Dosing Q3W 21-day cycle Dosing Q3W N=30 N=30 MTD MTD RP2D RP2D STRO 001
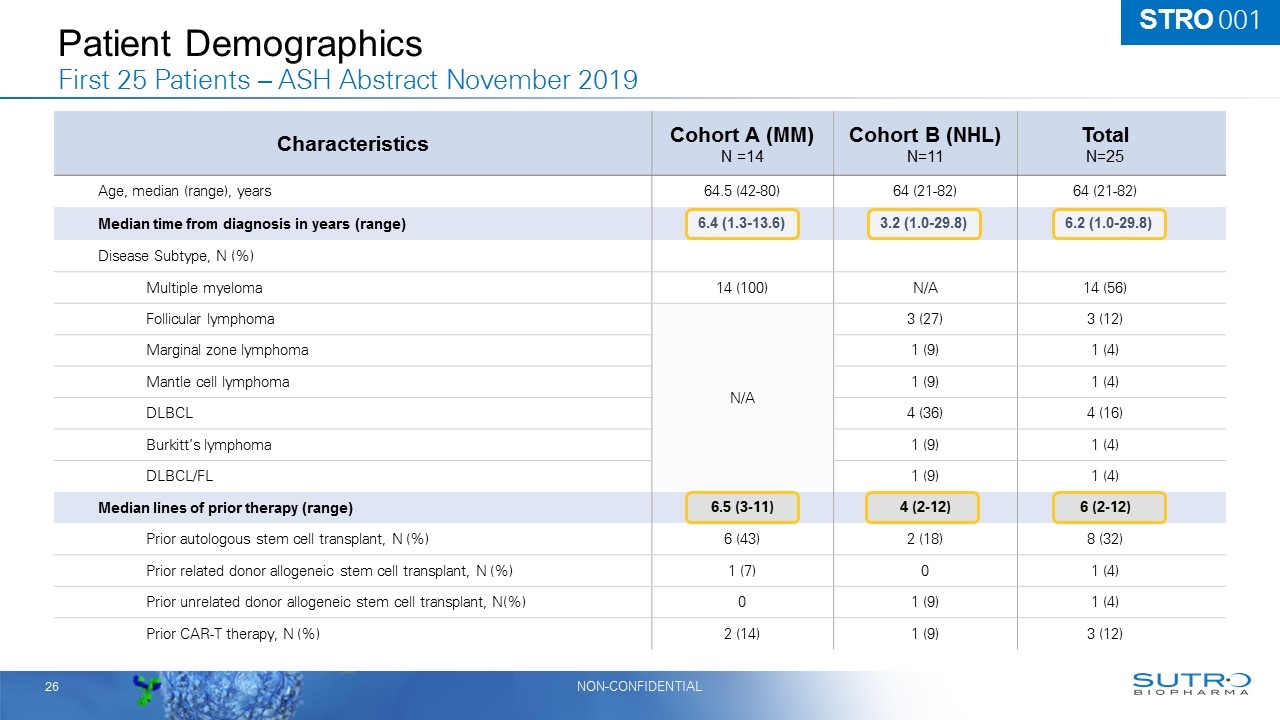
Patient Demographics First 25 Patients – ASH Abstract November 2019 Characteristics Cohort A (MM) N =14 Cohort B (NHL) N=11 Total N=25 Age, median (range), years 64.5 (42-80) 64 (21-82) 64 (21-82) Median time from diagnosis in years (range) Disease Subtype, N (%) Multiple myeloma 14 (100) N/A 14 (56) Follicular lymphoma N/A 3 (27) 3 (12) Marginal zone lymphoma 1 (9) 1 (4) Mantle cell lymphoma 1 (9) 1 (4) DLBCL 4 (36) 4 (16) Burkitt’s lymphoma 1 (9) 1 (4) DLBCL/FL 1 (9) 1 (4) Median lines of prior therapy (range) 6.5 (3-11) 4 (2-12) 6 (2-12) Prior autologous stem cell transplant, N (%) 6 (43) 2 (18) 8 (32) Prior related donor allogeneic stem cell transplant, N (%) 1 (7) 0 1 (4) Prior unrelated donor allogeneic stem cell transplant, N(%) 0 1 (9) 1 (4) Prior CAR-T therapy, N (%) 2 (14) 1 (9) 3 (12) STRO 001 6.4 (1.3-13.6) 3.2 (1.0-29.8) 6.2 (1.0-29.8)

Generally Well Tolerated with Early Signs of Anti-Tumor Activity Presented at EHA June 2019 and Updated in ASH Abstract November 2019 STRO 001 STRO-001 was generally well tolerated, most AEs were Grade 1 & 2 No ocular toxicity signals have been observed Preliminary anti-tumor activity: DLBCL: 1CR & 1PR MM: 1 Stable disease Initiated screening for pre-existing thromboses and if discovered, patient administered anticoagulants before study commencement. No new thromboembolisms observed subsequent to screening
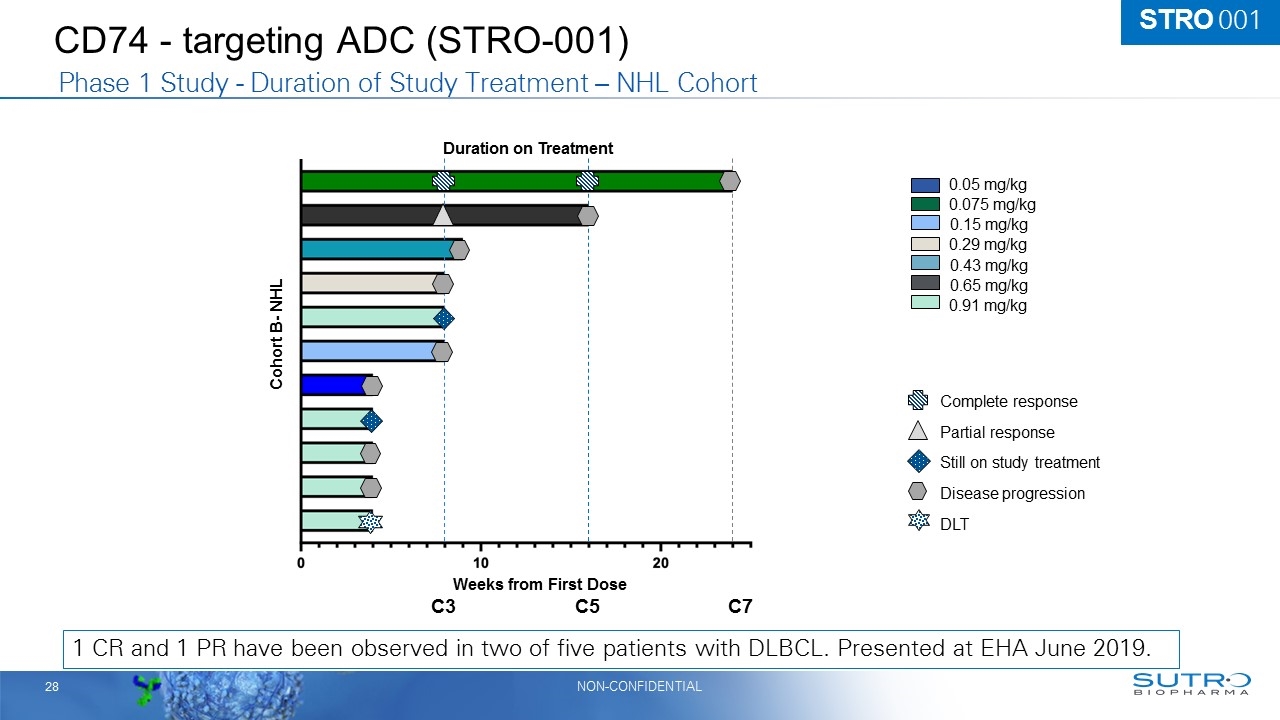
CD74 - targeting ADC (STRO-001) 1 CR and 1 PR have been observed in two of five patients with DLBCL. Presented at EHA June 2019. 0.05 mg/kg 0.075 mg/kg 0.15 mg/kg 0.29 mg/kg 0.43 mg/kg 0.65 mg/kg 0.91 mg/kg Still on study treatment DLT Disease progression Complete response Partial response Cohort B- NHL Weeks from First Dose Duration on Treatment Phase 1 Study - Duration of Study Treatment – NHL Cohort C3 C7 C5 STRO 001
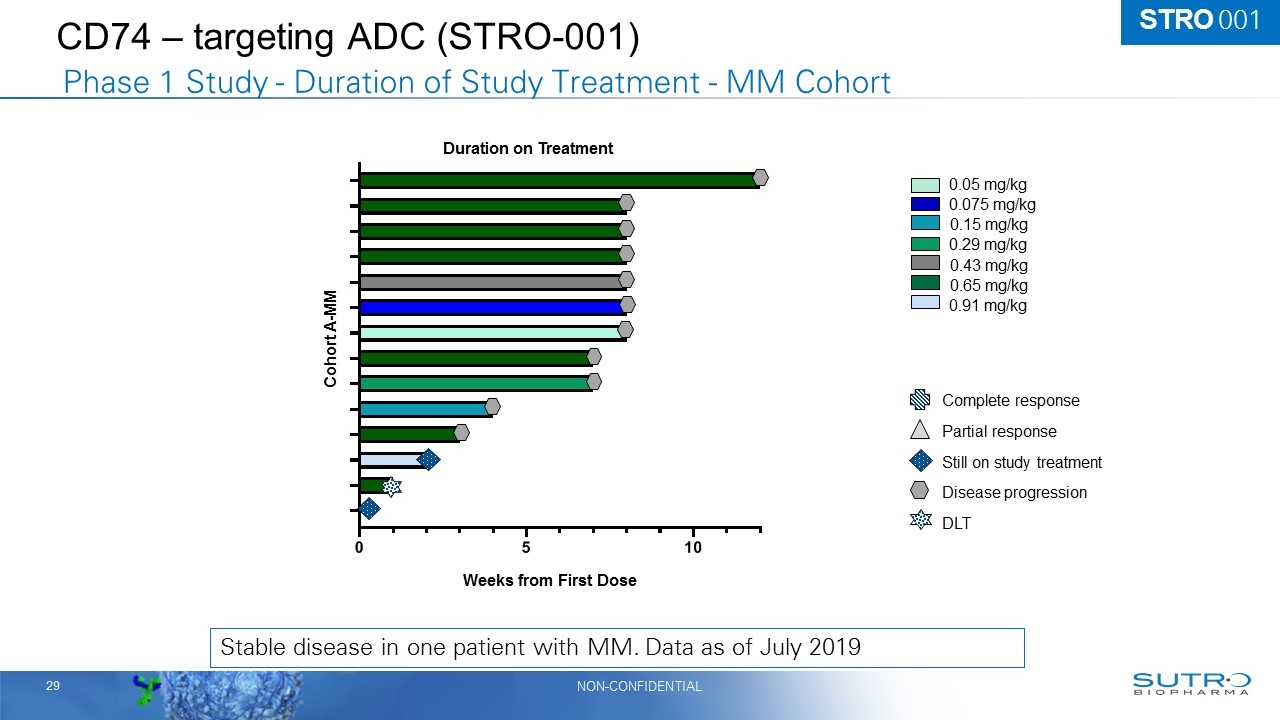
CD74 – targeting ADC (STRO-001) Stable disease in one patient with MM. Data as of July 2019 0.05 mg/kg 0.075 mg/kg 0.15 mg/kg 0.29 mg/kg 0.43 mg/kg 0.65 mg/kg 0.91 mg/kg Still on study treatment DLT Disease progression Complete response Partial response Cohort A-MM Duration on Treatment Weeks from First Dose Phase 1 Study - Duration of Study Treatment - MM Cohort STRO 001
CC–99712 (BCMA ADC) Phase 1 Study Enrolling Patients with Relapsed Refractory Multiple Myeloma CC 99712 BCMA – targeting ADC Potentially Available in Community Oncology Centers CC-99712 was discovered and developed using Sutro’s XpressCF® Source: Bristol-Myers Squibb ASH 2019. BCMA ADC CC-99712 BCMA – Validated Target in Multiple Myeloma BCMA CAR-T ide-cel (bb2121) orva-cel (JCARH125) bb21217 BCMA TCE CC-93269
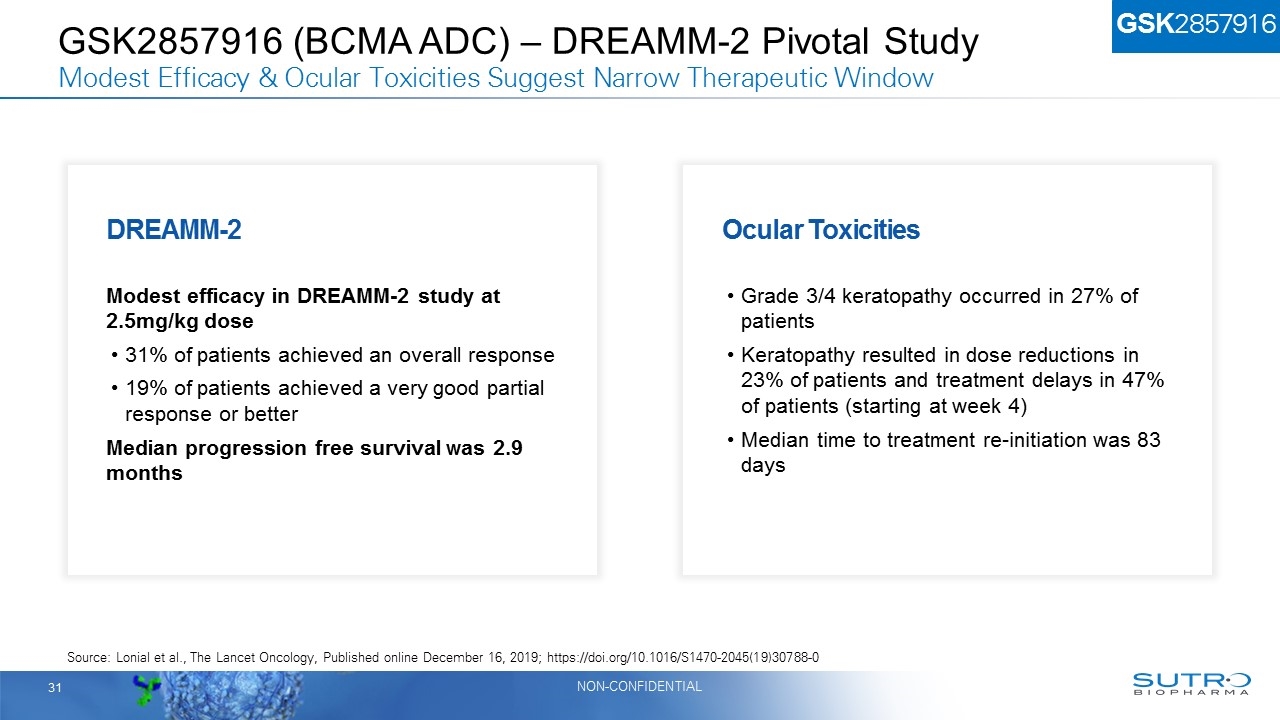
GSK2857916 (BCMA ADC) – DREAMM-2 Pivotal Study Modest Efficacy & Ocular Toxicities Suggest Narrow Therapeutic Window Ocular Toxicities Grade 3/4 keratopathy occurred in 27% of patients Keratopathy resulted in dose reductions in 23% of patients and treatment delays in 47% of patients (starting at week 4) Median time to treatment re-initiation was 83 days DREAMM-2 Modest efficacy in DREAMM-2 study at 2.5mg/kg dose 31% of patients achieved an overall response 19% of patients achieved a very good partial response or better Median progression free survival was 2.9 months Source: Lonial et al., The Lancet Oncology, Published online December 16, 2019; https://doi.org/10.1016/S1470-2045(19)30788-0 GSK2857916
Sutro’s Next Generation Tumor Targeting Immunostimulatory ADC Breakthrough technology for dual conjugated immunostimulatory antibody drug conjugate Designed and enabled using Sutro’s XpressCF+TM platform Enables simultaneous and precise tumor targeting of a cytotoxin and a novel toll-like receptor (TLR) agonist with systemic delivery Novel design intended to prime an adaptive anti-tumor response in a monotherapy Potential to reprogram the patient’s tumor microenvironment and generate protective anti-tumor immunity Data Presented at the World ADC Meeting in London, 3/2020 iADC – Off the shelf, systemically administered in situ immunization
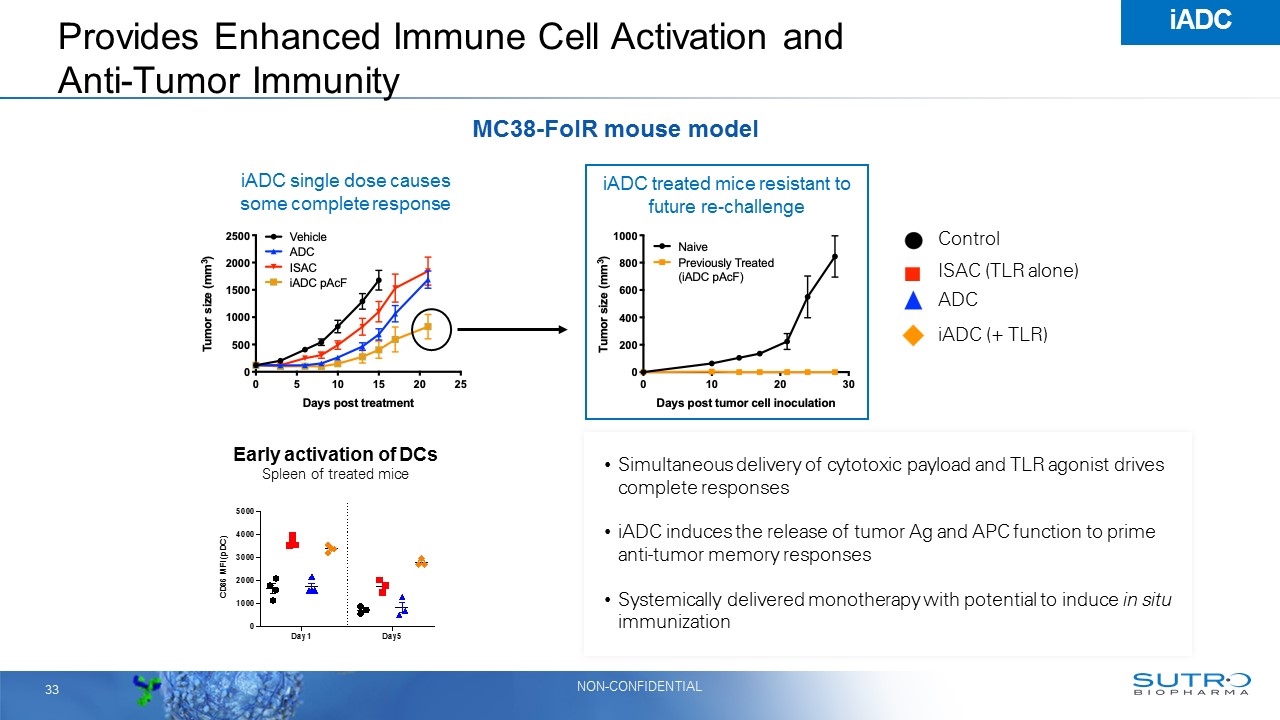
Provides Enhanced Immune Cell Activation and Anti-Tumor Immunity Early activation of DCs Spleen of treated mice Control iADC (+ TLR) ADC ISAC (TLR alone) iADC single dose causes some complete response MC38-FolR mouse model iADC treated mice resistant to future re-challenge Simultaneous delivery of cytotoxic payload and TLR agonist drives complete responses iADC induces the release of tumor Ag and APC function to prime anti-tumor memory responses Systemically delivered monotherapy with potential to induce in situ immunization iADC
Experienced Leadership Team William Newell, JD Chief Executive Officer and Member of the Board of Directors Trevor Hallam, PhD Chief Scientific Officer Arturo Molina, MD, MS, FACP Chief Medical Officer Ed Albini Chief Financial Officer Shabbir Anik, PhD Chief Technical Operations Officer Linda Fitzpatrick Chief People and Communications Officer Nicki Vasquez, PhD Sr. VP Alliance Management / Portfolio Strategy & Operations Lynx Neurex

Company Overview June 2020 NASDAQ: STRO Bill Newell, CEO