EX-99.2
Published on April 27, 2020

NASDAQ: STRO Bill Newell, CEO Sutro Biopharma Analyst and Investor Conference Call April 27, 2020 Exhibit 99.2

Forward Looking Statements This presentation and the accompanying oral presentation contain “forward-looking” statements that are based on our management’s beliefs and assumptions and on information currently available to management. Forward-looking statements include all statements other than statements of historical fact contained in this presentation, including information concerning our future financial performance, business plans and objectives, current and future clinical and preclinical activities, timing and success of our ongoing and planned clinical trials and related data, the timing of announcements, updates and results of our clinical trials and related data, timing and success of our planned development activities, our ability to obtain and maintain regulatory approval, the potential therapeutic benefits and economic value of our product candidates, potential growth opportunities, financing plans, competitive position, industry environment and potential market opportunities. Forward-looking statements are subject to known and unknown risks, uncertainties, assumptions and other factors, including risks and uncertainties related to our cash forecasts, our and our collaborators’ ability to advance our product candidates, the receipt and timing of potential regulatory submissions, designations, approvals and commercialization of product candidates, the timing and results of preclinical and clinical trials, and the expected impact of the COVID-19 pandemic on our operations. It is not possible for our management to predict all risks, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements we may make. These factors, together with those that may be described in greater detail under the heading “Risk Factors” contained in our most recent Annual Report on Form 10-K and other reports the company files from time to time with the Securities and Exchange Commission, may cause our actual results, performance or achievements to differ materially and adversely from those anticipated or implied by our forward-looking statements. You should not rely upon forward-looking statements as predictions of future events. Although our management believes that the expectations reflected in our forward-looking statements are reasonable, we cannot guarantee that the future results, levels of activity, performance or events and circumstances described in the forward-looking statements will be achieved or occur. Moreover, neither we nor our management assume responsibility for the accuracy and completeness of the forward-looking statements. We undertake no obligation to publicly update any forward-looking statements for any reason after the date of this presentation to conform these statements to actual results or to changes in our expectations, except as required by law. This presentation also contains estimates and other statistical data made by independent parties and by us relating to market size and growth and other data about our industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. In addition, projections, assumptions, and estimates of our future performance and the future performance of the markets in which we operate are necessarily subject to a high degree of uncertainty and risk. Solely for convenience, the trademarks and tradenames referred to in this presentation appear without the ® and ™ symbols, but those references are not intended to indicate, in any way, that we will not assert, to the fullest extent under applicable law, our rights, or the right of the applicable licensor to these trademarks and tradenames. CONFIDENTIAL
CONFIDENTIAL STRO-002-GM1, a First in Human, Phase 1 study of STRO-002, an anti-Folate Receptor-alpha (FRα) Antibody Drug Conjugate, in Patients with Advanced Platinum Resistant/Refractory Epithelial Ovarian Cancer, including Fallopian Tube or Primary Peritoneal Cancers R. Wendel Naumann, Denise Uyar, John W. Moroney, Fadi S. Braiteh, Russell J. Schilder, John P. Diaz, Erika Hamilton, Sami Diab, Lainie P. Martin, David M. O'Malley, Richard T. Penson, Clifford DiLea, Michael Palumbo, Venita De Almeida, Shannon Matheny, Arturo Molina.
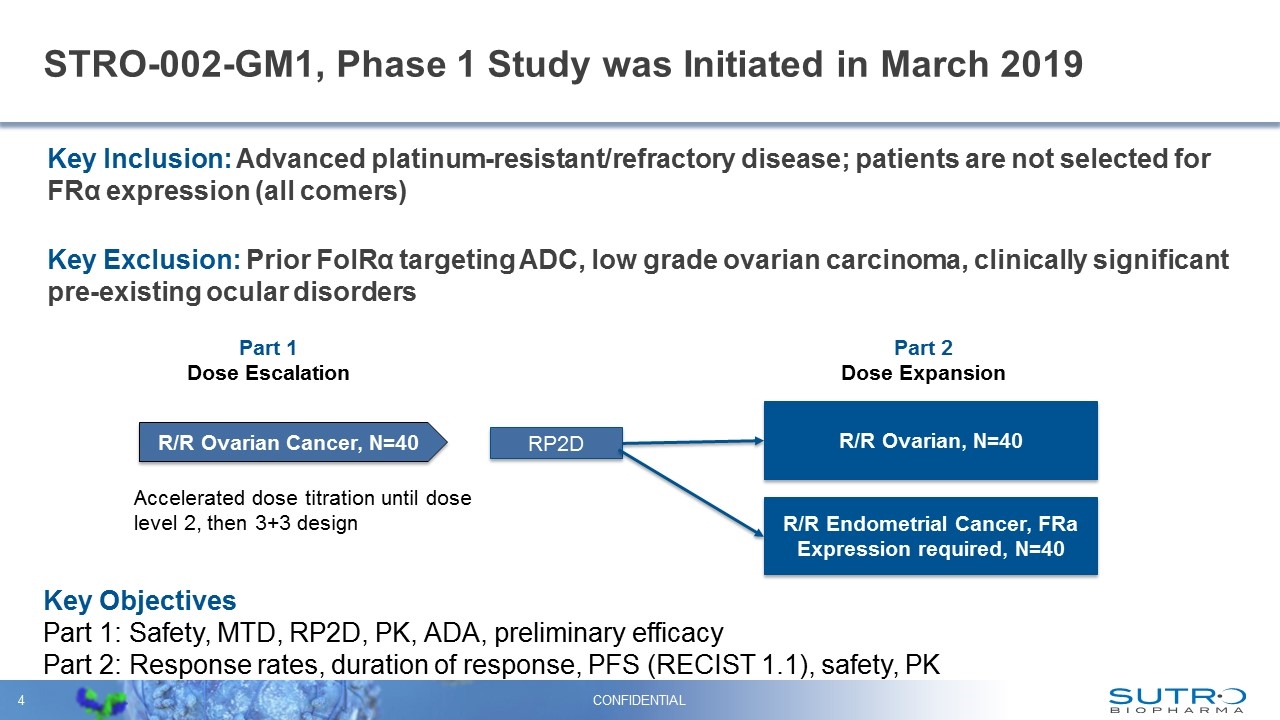
STRO-002-GM1, Phase 1 Study was Initiated in March 2019 Key Inclusion: Advanced platinum-resistant/refractory disease; patients are not selected for FRα expression (all comers) Key Exclusion: Prior FolRα targeting ADC, low grade ovarian carcinoma, clinically significant pre-existing ocular disorders CONFIDENTIAL Part 1 Dose Escalation R/R Ovarian Cancer, N=40 RP2D R/R Ovarian, N=40 R/R Endometrial Cancer, FRa Expression required, N=40 Part 2 Dose Expansion Accelerated dose titration until dose level 2, then 3+3 design Key Objectives Part 1: Safety, MTD, RP2D, PK, ADA, preliminary efficacy Part 2: Response rates, duration of response, PFS (RECIST 1.1), safety, PK
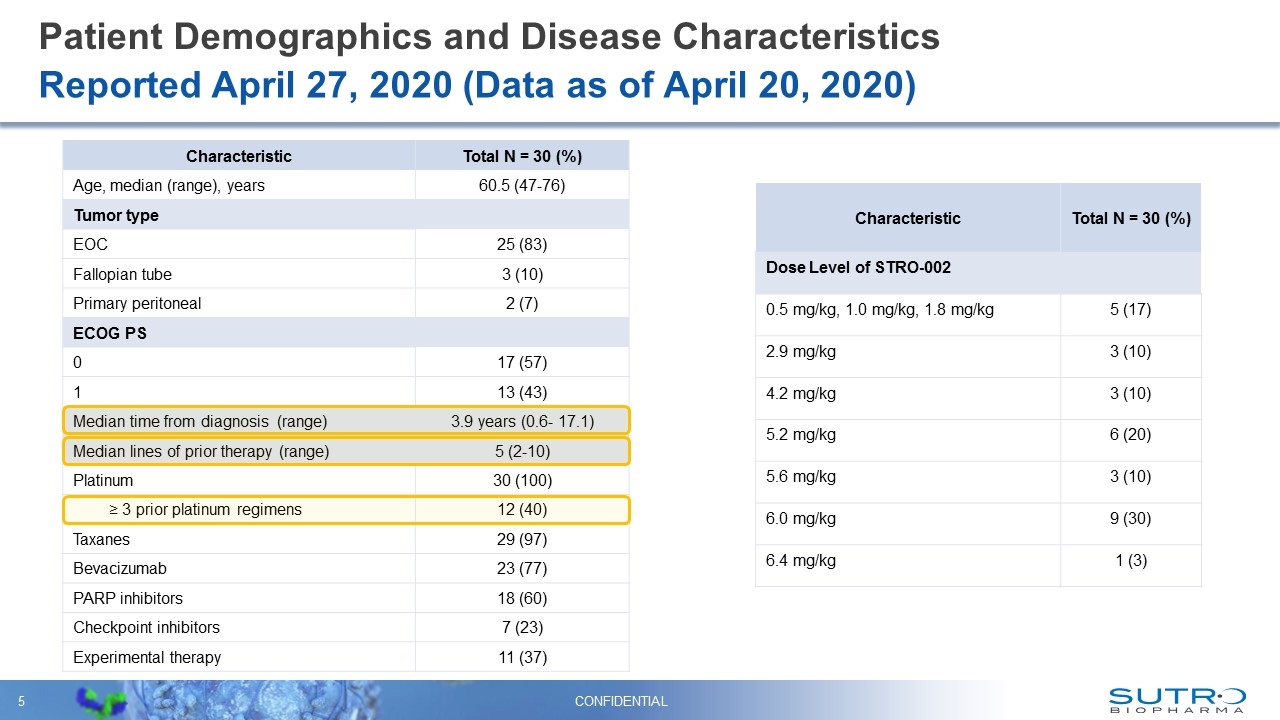
Patient Demographics and Disease Characteristics CONFIDENTIAL Reported April 27, 2020 (Data as of April 20, 2020) Characteristic Total N = 30 (%) Age, median (range), years 60.5 (47-76) Tumor type EOC 25 (83) Fallopian tube 3 (10) Primary peritoneal 2 (7) ECOG PS 0 17 (57) 1 13 (43) Median time from diagnosis (range) 3.9 years (0.6- 17.1) Median lines of prior therapy (range) 5 (2-10) Platinum 30 (100) ≥ 3 prior platinum regimens 12 (40) Taxanes 29 (97) Bevacizumab 23 (77) PARP inhibitors 18 (60) Checkpoint inhibitors 7 (23) Experimental therapy 11 (37) Characteristic Total N = 30 (%) Dose Level of STRO-002 0.5 mg/kg, 1.0 mg/kg, 1.8 mg/kg 5 (17) 2.9 mg/kg 3 (10) 4.2 mg/kg 3 (10) 5.2 mg/kg 6 (20) 5.6 mg/kg 3 (10) 6.0 mg/kg 9 (30) 6.4 mg/kg 1 (3)
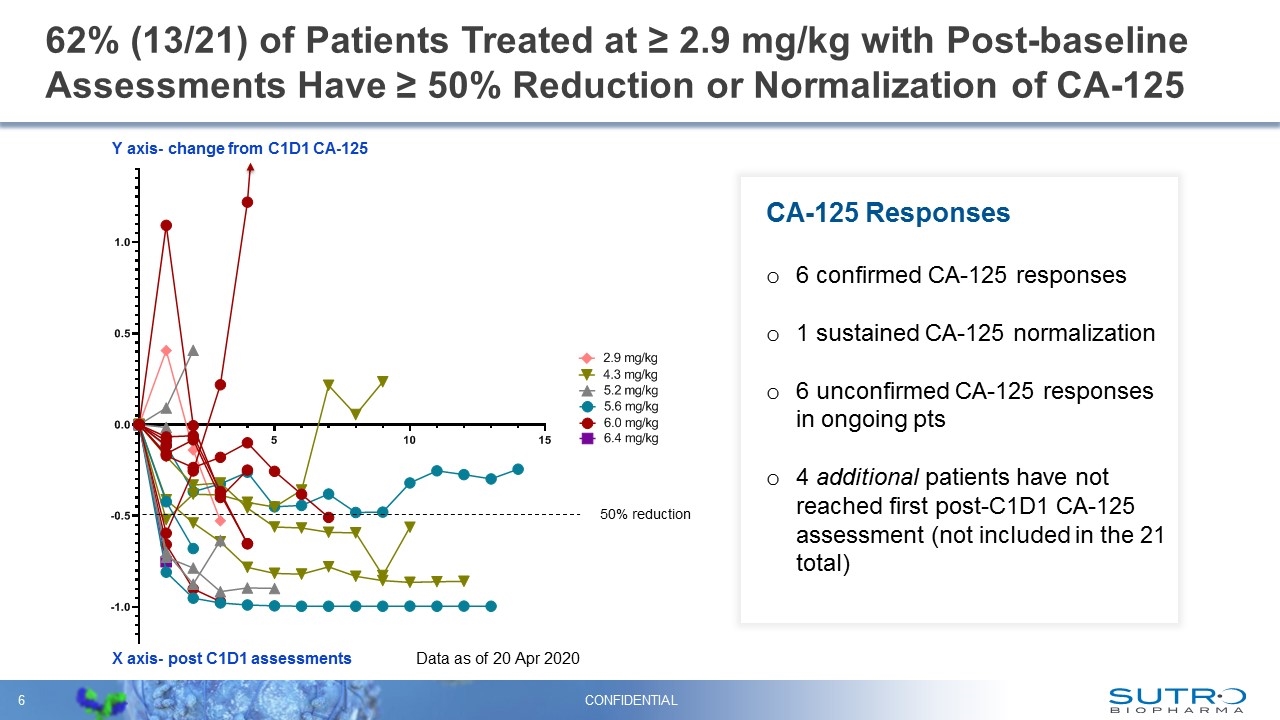
62% (13/21) of Patients Treated at ≥ 2.9 mg/kg with Post-baseline Assessments Have ≥ 50% Reduction or Normalization of CA-125 CONFIDENTIAL 50% reduction X axis- post C1D1 assessments Y axis- change from C1D1 CA-125 Data as of 20 Apr 2020 CA-125 Responses 6 confirmed CA-125 responses 1 sustained CA-125 normalization 6 unconfirmed CA-125 responses in ongoing pts 4 additional patients have not reached first post-C1D1 CA-125 assessment (not included in the 21 total)
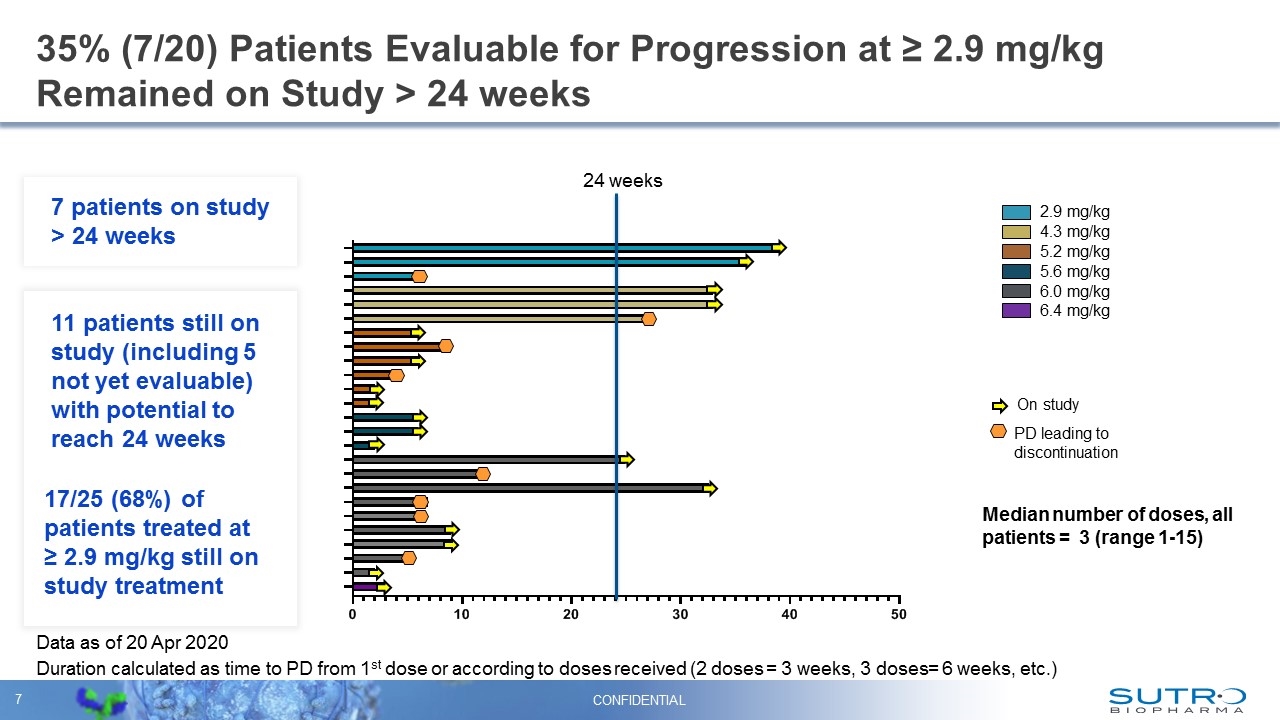
35% (7/20) Patients Evaluable for Progression at ≥ 2.9 mg/kg Remained on Study > 24 weeks 2.9 mg/kg 4.3 mg/kg 6.0 mg/kg Duration calculated as time to PD from 1st dose or according to doses received (2 doses = 3 weeks, 3 doses= 6 weeks, etc.) 5.2 mg/kg 5.6 mg/kg 6.4 mg/kg On study PD leading to discontinuation Median number of doses, all patients = 3 (range 1-15) Data as of 20 Apr 2020 CONFIDENTIAL 11 patients still on study (including 5 not yet evaluable) with potential to reach 24 weeks 17/25 (68%) of patients treated at ≥ 2.9 mg/kg still on study treatment 24 weeks 7 patients on study > 24 weeks
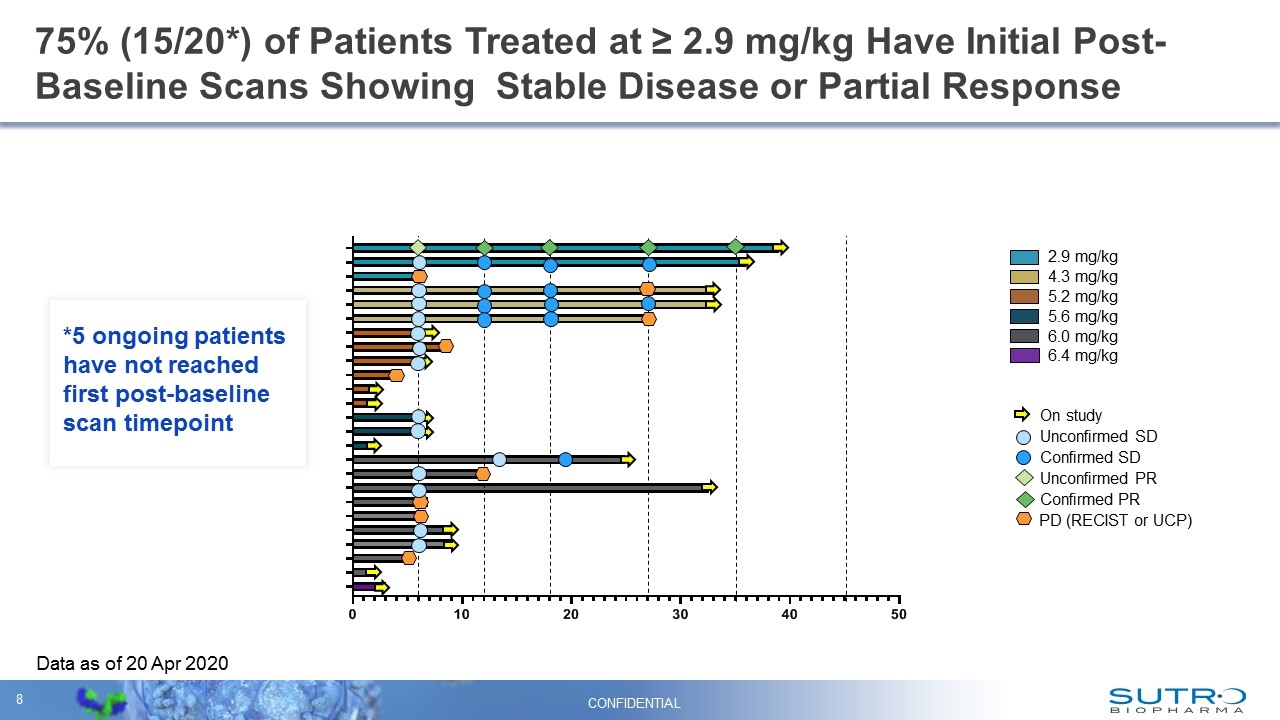
75% (15/20*) of Patients Treated at ≥ 2.9 mg/kg Have Initial Post-Baseline Scans Showing Stable Disease or Partial Response 2.9 mg/kg 4.3 mg/kg 6.0 mg/kg 5.2 mg/kg 5.6 mg/kg 6.4 mg/kg On study Confirmed PR Unconfirmed SD Confirmed SD Unconfirmed PR PD (RECIST or UCP) Data as of 20 Apr 2020 CONFIDENTIAL On study On study On study On study On study On study On study On study On study On study On study On study On study On study On study On study On study *5 ongoing patients have not reached first post-baseline scan timepoint
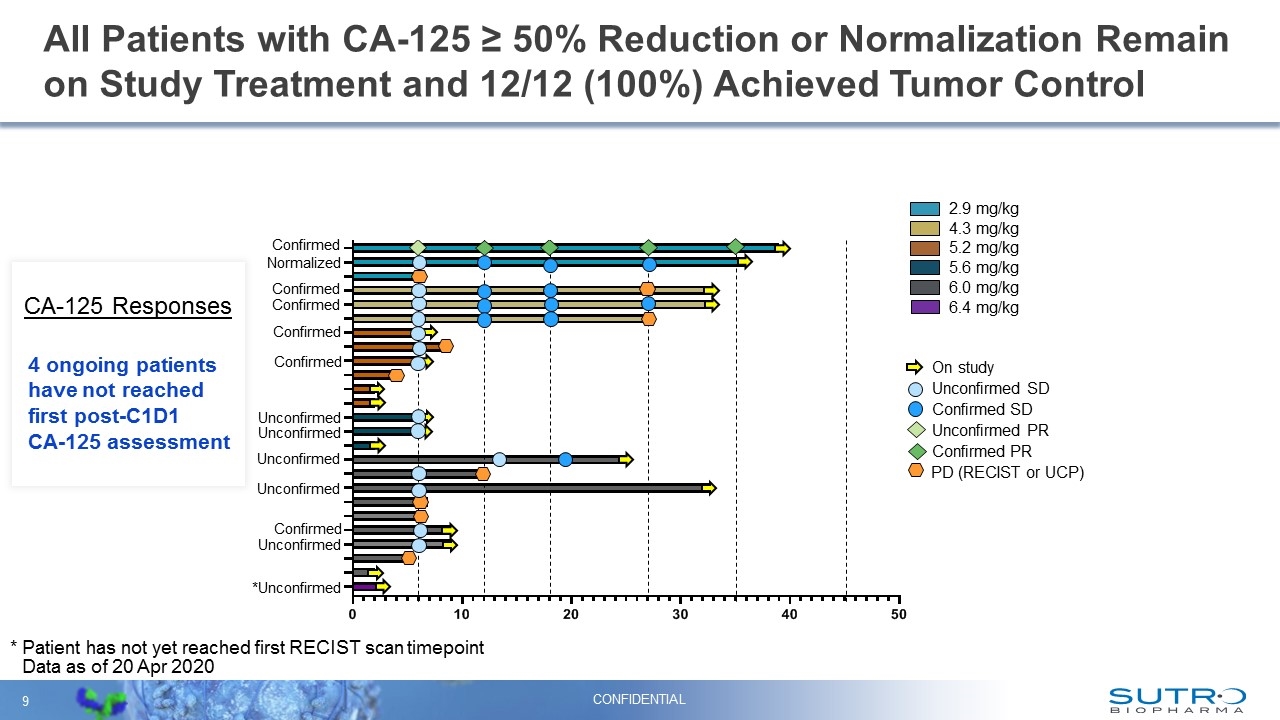
All Patients with CA-125 ≥ 50% Reduction or Normalization Remain on Study Treatment and 12/12 (100%) Achieved Tumor Control 2.9 mg/kg 4.3 mg/kg 6.0 mg/kg 5.2 mg/kg 5.6 mg/kg 6.4 mg/kg On study Confirmed PR Unconfirmed SD Confirmed SD Unconfirmed PR PD (RECIST or UCP) Data as of 20 Apr 2020 Confirmed Unconfirmed Unconfirmed Confirmed Confirmed Confirmed Confirmed Normalized Unconfirmed Unconfirmed *Unconfirmed Unconfirmed CONFIDENTIAL 4 ongoing patients have not reached first post-C1D1 CA-125 assessment CA-125 Responses Confirmed * Patient has not yet reached first RECIST scan timepoint
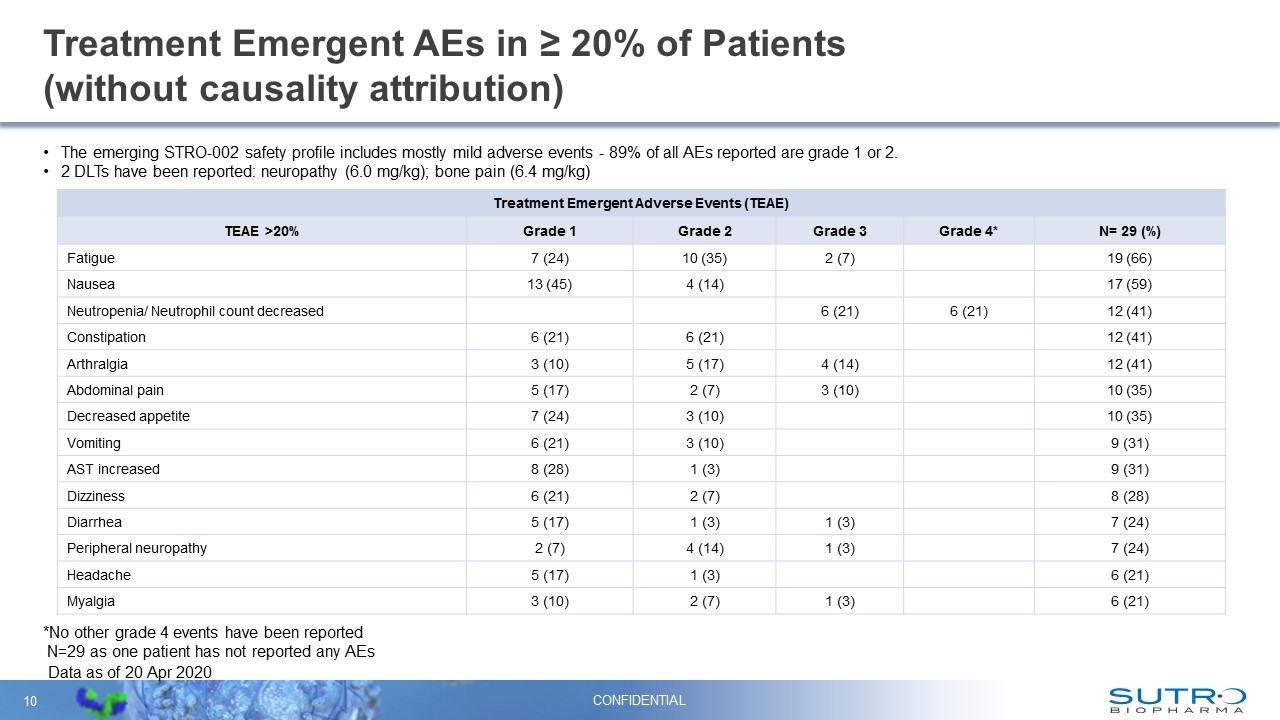
Treatment Emergent AEs in ≥ 20% of Patients (without causality attribution) Data as of 20 Apr 2020 Treatment Emergent Adverse Events (TEAE) TEAE >20% Grade 1 Grade 2 Grade 3 Grade 4* N= 29 (%) Fatigue 7 (24) 10 (35) 2 (7) 19 (66) Nausea 13 (45) 4 (14) 17 (59) Neutropenia/ Neutrophil count decreased 6 (21) 6 (21) 12 (41) Constipation 6 (21) 6 (21) 12 (41) Arthralgia 3 (10) 5 (17) 4 (14) 12 (41) Abdominal pain 5 (17) 2 (7) 3 (10) 10 (35) Decreased appetite 7 (24) 3 (10) 10 (35) Vomiting 6 (21) 3 (10) 9 (31) AST increased 8 (28) 1 (3) 9 (31) Dizziness 6 (21) 2 (7) 8 (28) Diarrhea 5 (17) 1 (3) 1 (3) 7 (24) Peripheral neuropathy 2 (7) 4 (14) 1 (3) 7 (24) Headache 5 (17) 1 (3) 6 (21) Myalgia 3 (10) 2 (7) 1 (3) 6 (21) The emerging STRO-002 safety profile includes mostly mild adverse events - 89% of all AEs reported are grade 1 or 2. 2 DLTs have been reported: neuropathy (6.0 mg/kg); bone pain (6.4 mg/kg) *No other grade 4 events have been reported N=29 as one patient has not reported any AEs CONFIDENTIAL
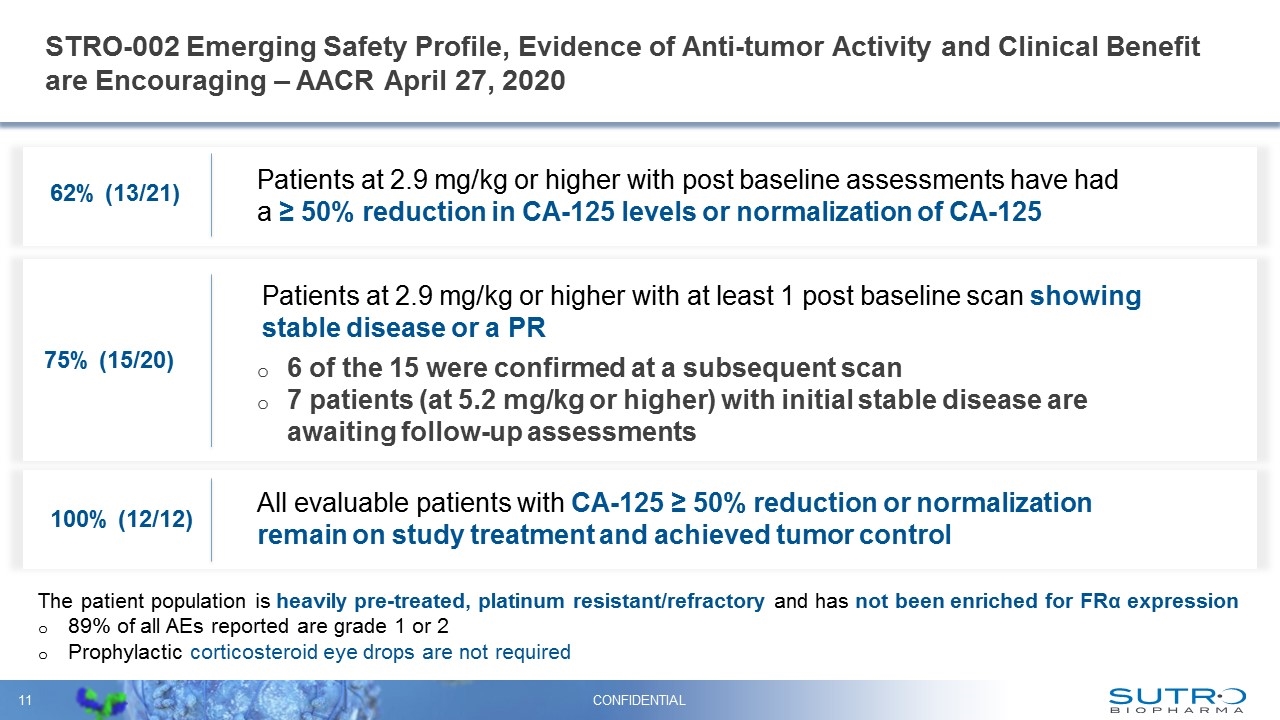
STRO-002 Emerging Safety Profile, Evidence of Anti-tumor Activity and Clinical Benefit are Encouraging – AACR April 27, 2020 CONFIDENTIAL 62% (13/21) Patients at 2.9 mg/kg or higher with post baseline assessments have had a ≥ 50% reduction in CA-125 levels or normalization of CA-125 75% (15/20) Patients at 2.9 mg/kg or higher with at least 1 post baseline scan showing stable disease or a PR 6 of the 15 were confirmed at a subsequent scan 7 patients (at 5.2 mg/kg or higher) with initial stable disease are awaiting follow-up assessments 100% (12/12) All evaluable patients with CA-125 ≥ 50% reduction or normalization remain on study treatment and achieved tumor control The patient population is heavily pre-treated, platinum resistant/refractory and has not been enriched for FRα expression 89% of all AEs reported are grade 1 or 2 Prophylactic corticosteroid eye drops are not required

Summary - Well Tolerated, Encouraging Clinical Benefit Expansion Cohorts Planned for 2H20 CONFIDENTIAL STRO-002 was generally well tolerated and mostly associated with mild events 5/30= 17% have not had post-treatment scan for initial RECIST assessment 89% of all AEs reported are grade 1 or 2 Prophylactic corticosteroid eye drops have not been required MTD has not been reached, additional patients are being enrolled in the 5.2mg/kg – 6.0mg/kg range to better characterize RP2D Follow-up is still early and enrollment ongoing 5/30 = 17% have not had post-treatment scan for initial RECIST assessment Next Steps: Recommended Phase 2 dose to be confirmed Expansion cohorts to be initiated

Thank You to the Patients, their Families and our Participating Study Site Investigators and Staff CONFIDENTIAL Levine Cancer Institute, Carolinas Medical Center, Charlotte, NC Medical College of Wisconsin, Milwaukee, WI University of Chicago, Chicago, IL Comprehensive Cancer Centers of Nevada, Las Vegas, NV Sidney Kimmel Cancer Center, Thomas Jefferson University, Philadelphia, PA Miami Cancer Institute at Baptist Health, Miami, FL Sarah Cannon Research Institute, Tennessee Oncology PLLC, Nashville, TN Rocky Mountain Cancer Center, Aurora, CO University of Pennsylvania, Abramson Cancer Center, Philadelphia, PA Ohio State University, Wexner Medical Center, Columbus, OH Massachusetts General Hospital, Boston, MA

NASDAQ: STRO Bill Newell, CEO Sutro Biopharma Analyst and Investor Conference Call April 27, 2020