EX-99.3
Published on January 9, 2023
Luveltamab Tazevibulin(Luvelta, STRO-002) Phase 1 Data and Regulatory Strategy January 9, 2023 Exhibit 99.3

Agenda for TodayJanuary 9, 2023 Topic Speaker Welcome and Introduction Forward-Looking Statements Ed Albini, Chief Financial Officer, Sutro Biopharma Bill Newell, Chief Executive Officer, Sutro Biopharma Luvelta (STRO-002) Phase 1 Dose-Expansion Study Results Dr. R. Wendel Naumann, Professor and Director of Gynecologic Oncology Research, Associate Medical Director of Clinical Trials at the Levine Cancer Institute, Atrium Health Registrational Path Forward for Luvelta Bill Newell Dr. Stan Frankel, Scientific Advisory Board member, Sutro Biopharma Market Opportunity for Ovarian Cancer Treatment Bill NewellJane Chung, Chief Commercial Officer, Sutro Biopharma Closing Remarks Bill Newell Q&A Bill NewellDr. R. Wendel Naumann Dr. Stan Frankel Trevor Hallam, President, Research & Chief Scientific Officer, Sutro Biopharma Jane Chung

Forward-Looking Statements This presentation and the accompanying oral presentation contain “forward-looking” statements that are based on our management’s beliefs and assumptions and on information currently available to management. Forward-looking statements include all statements other than statements of historical fact contained in this presentation, including information concerning our future financial performance, business plans and objectives, current and future clinical activities, timing, design and success of our ongoing and planned clinical trials and related data, updates and results of our clinical trials and related data, timing and success of our planned development activities, our ability to obtain and maintain regulatory approval, the potential therapeutic benefits and economic value of our product candidates, potential growth opportunities, financing plans, potential future milestone and royalty payments, competitive position, industry environment and potential market opportunities for the Company’s product candidates. Forward-looking statements are subject to known and unknown risks, uncertainties, assumptions and other factors, including risks and uncertainties related to our cash forecasts, our and our collaborators’ ability to advance our product candidates, the receipt, feedback and timing of potential regulatory submissions, designations, approvals and commercialization of product candidates, the design, timing and results of preclinical and clinical trials, and the expected impact of the COVID-19 pandemic on our operations. It is not possible for our management to predict all risks, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements we may make. These factors, together with those that may be described in greater detail under the heading “Risk Factors” contained in our most recent Annual Report on Form 10-K, Quarterly Report on Form 10-Q and other reports the company files from time to time with the Securities and Exchange Commission, may cause our actual results, performance or achievements to differ materially and adversely from those anticipated or implied by our forward-looking statements. You should not rely upon forward-looking statements as predictions of future events. Although our management believes that the expectations reflected in our forward-looking statements are reasonable, we cannot guarantee that the future results, levels of activity, performance or events and circumstances described in the forward-looking statements will be achieved or occur. Moreover, neither we nor our management assume responsibility for the accuracy and completeness of the forward-looking statements. We undertake no obligation to publicly update any forward-looking statements for any reason after the date of this presentation to conform these statements to actual results or to changes in our expectations, except as required by law. This presentation also contains estimates and other statistical data made by independent parties and by us relating to market size and growth and other data about our industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. In addition, projections, assumptions, and estimates of our future performance and the future performance of the markets in which we operate are necessarily subject to a high degree of uncertainty and risk.

Luveltamab Tazevibulin (Luvelta, STRO-002) Phase 1 Dose-Expansion Study
Advanced Ovarian Cancer Has a High Unmet Medical Need Ovarian cancer is the most common cause of death from gynecological cancers Accounts for 2.1% of all estimated cancer deaths(1,2) Almost half of affected women live less than five years following diagnosis1,2 In 2022, an estimated 19,880 new ovarian cancer cases were diagnosed in the United States(1,2) Total estimated death from this disease was 12,810 Folate receptor alpha, or FolRα is highly expressed in ovarian cancer Associated with disease burden and treatment outcomes(3,4) Due to advanced stage of disease at diagnosis and limited progress of available treatments FolRα, folate receptor alpha. 1. Cancer facts and figures 2022. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2022/2022-cancer-facts-and-figures.pdf. Accessed December 14, 2022. 2. 2022 Estimates. American Cancer Society. https://cancerstatisticscenter.cancer.org/?_ga=2.9856755.798860474.1671221534-46877757.1671052212#!/. Accessed December 16, 2022. 3. Birrer MJ, et al. Oncologist. 2019;24:425–429. 3. https://www.nature.com/articles/s41416-022-02031-x Z FolRα FolRα FolRα
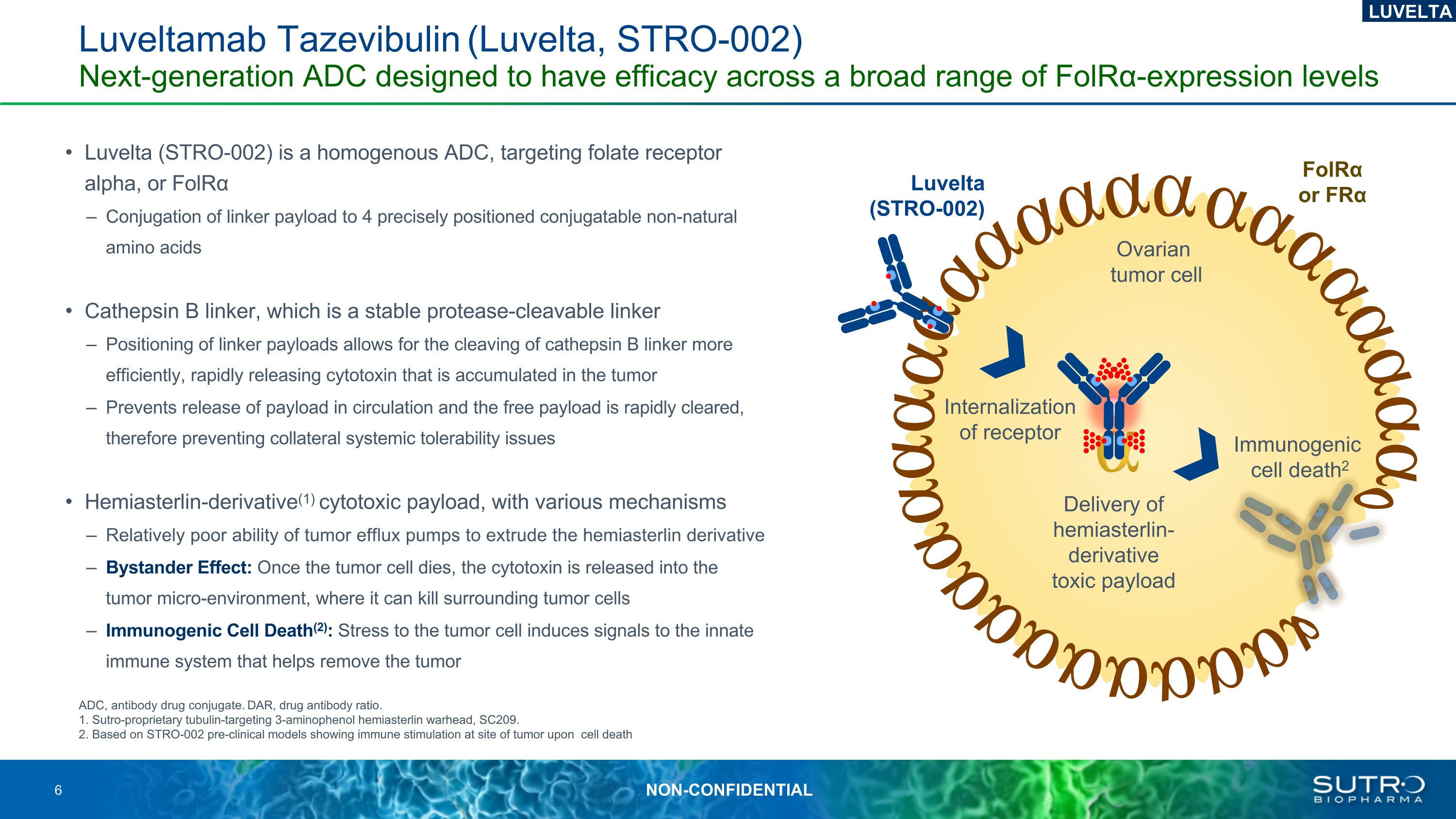
Luveltamab Tazevibulin (Luvelta, STRO-002) Next-generation ADC designed to have efficacy across a broad range of FolRα-expression levels ADC, antibody drug conjugate. DAR, drug antibody ratio. 1. Sutro-proprietary tubulin-targeting 3-aminophenol hemiasterlin warhead, SC209. 2. Based on STRO-002 pre-clinical models showing immune stimulation at site of tumor upon cell death Ovarian tumor cell FolRα or FRα Delivery of hemiasterlin-derivative toxic payload Internalization of receptor Immunogenic cell death2 Luvelta (STRO-002) LUVELTA Luvelta (STRO-002) is a homogenous ADC, targeting folate receptor alpha, or FolRα Conjugation of linker payload to 4 precisely positioned conjugatable non-natural amino acids Cathepsin B linker, which is a stable protease-cleavable linker Positioning of linker payloads allows for the cleaving of cathepsin B linker more efficiently, rapidly releasing cytotoxin that is accumulated in the tumor Prevents release of payload in circulation and the free payload is rapidly cleared, therefore preventing collateral systemic tolerability issues Hemiasterlin-derivative(1) cytotoxic payload, with various mechanisms Relatively poor ability of tumor efflux pumps to extrude the hemiasterlin derivative Bystander Effect: Once the tumor cell dies, the cytotoxin is released into the tumor micro-environment, where it can kill surrounding tumor cells Immunogenic Cell Death(2): Stress to the tumor cell induces signals to the innate immune system that helps remove the tumor
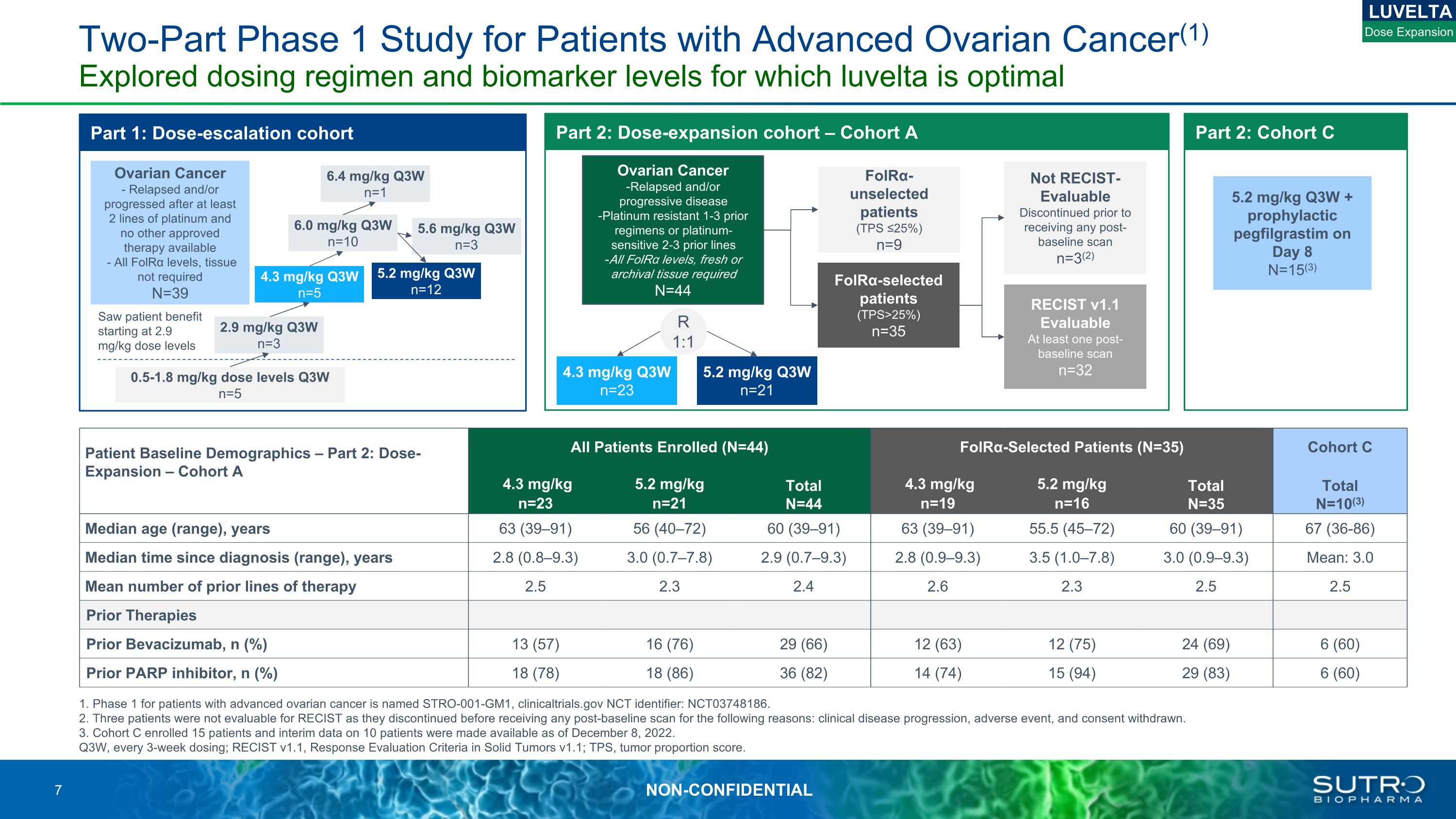
Two-Part Phase 1 Study for Patients with Advanced Ovarian Cancer(1) Explored dosing regimen and biomarker levels for which luvelta is optimal 1. Phase 1 for patients with advanced ovarian cancer is named STRO-001-GM1, clinicaltrials.gov NCT identifier: NCT03748186. 2. Three patients were not evaluable for RECIST as they discontinued before receiving any post-baseline scan for the following reasons: clinical disease progression, adverse event, and consent withdrawn. 3. Cohort C enrolled 15 patients and interim data on 10 patients were made available as of December 8, 2022. Q3W, every 3-week dosing; RECIST v1.1, Response Evaluation Criteria in Solid Tumors v1.1; TPS, tumor proportion score. Part 1: Dose-escalation cohort 5.6 mg/kg Q3W n=3 6.4 mg/kg Q3W n=1 5.2 mg/kg Q3W n=12 6.0 mg/kg Q3W n=10 4.3 mg/kg Q3W n=5 2.9 mg/kg Q3W n=3 0.5-1.8 mg/kg dose levels Q3W n=5 Part 2: Dose-expansion cohort – Cohort A 4.3 mg/kg Q3W n=23 RECIST v1.1 Evaluable At least one post-baseline scan n=32 Not RECIST-Evaluable Discontinued prior to receiving any post-baseline scan n=3(2) FolRα-unselected patients (TPS ≤25%) n=9 R 1:1 FolRα-selected patients (TPS>25%) n=35 5.2 mg/kg Q3W n=21 Part 2: Cohort C 5.2 mg/kg Q3W + prophylactic pegfilgrastim on Day 8 N=15(3) Ovarian Cancer Relapsed and/or progressive disease Platinum resistant 1-3 prior regimens or platinum-sensitive 2-3 prior lines All FolRα levels, fresh or archival tissue required N=44 LUVELTA Dose Expansion Saw patient benefit starting at 2.9 mg/kg dose levels Ovarian Cancer - Relapsed and/or progressed after at least 2 lines of platinum and no other approved therapy available - All FolRα levels, tissue not requiredN=39 Patient Baseline Demographics – Part 2: Dose-Expansion – Cohort A All Patients Enrolled (N=44) FolRα-Selected Patients (N=35) Cohort C 4.3 mg/kg n=23 5.2 mg/kg n=21 Total N=44 4.3 mg/kg n=19 5.2 mg/kg n=16 Total N=35 Total N=10(3) Median age (range), years 63 (39–91) 56 (40–72) 60 (39–91) 63 (39–91) 55.5 (45–72) 60 (39–91) 67 (36-86) Median time since diagnosis (range), years 2.8 (0.8–9.3) 3.0 (0.7–7.8) 2.9 (0.7–9.3) 2.8 (0.9–9.3) 3.5 (1.0–7.8) 3.0 (0.9–9.3) Mean: 3.0 Mean number of prior lines of therapy 2.5 2.3 2.4 2.6 2.3 2.5 2.5 Prior Therapies Prior Bevacizumab, n (%) 13 (57) 16 (76) 29 (66) 12 (63) 12 (75) 24 (69) 6 (60) Prior PARP inhibitor, n (%) 18 (78) 18 (86) 36 (82) 14 (74) 15 (94) 29 (83) 6 (60)
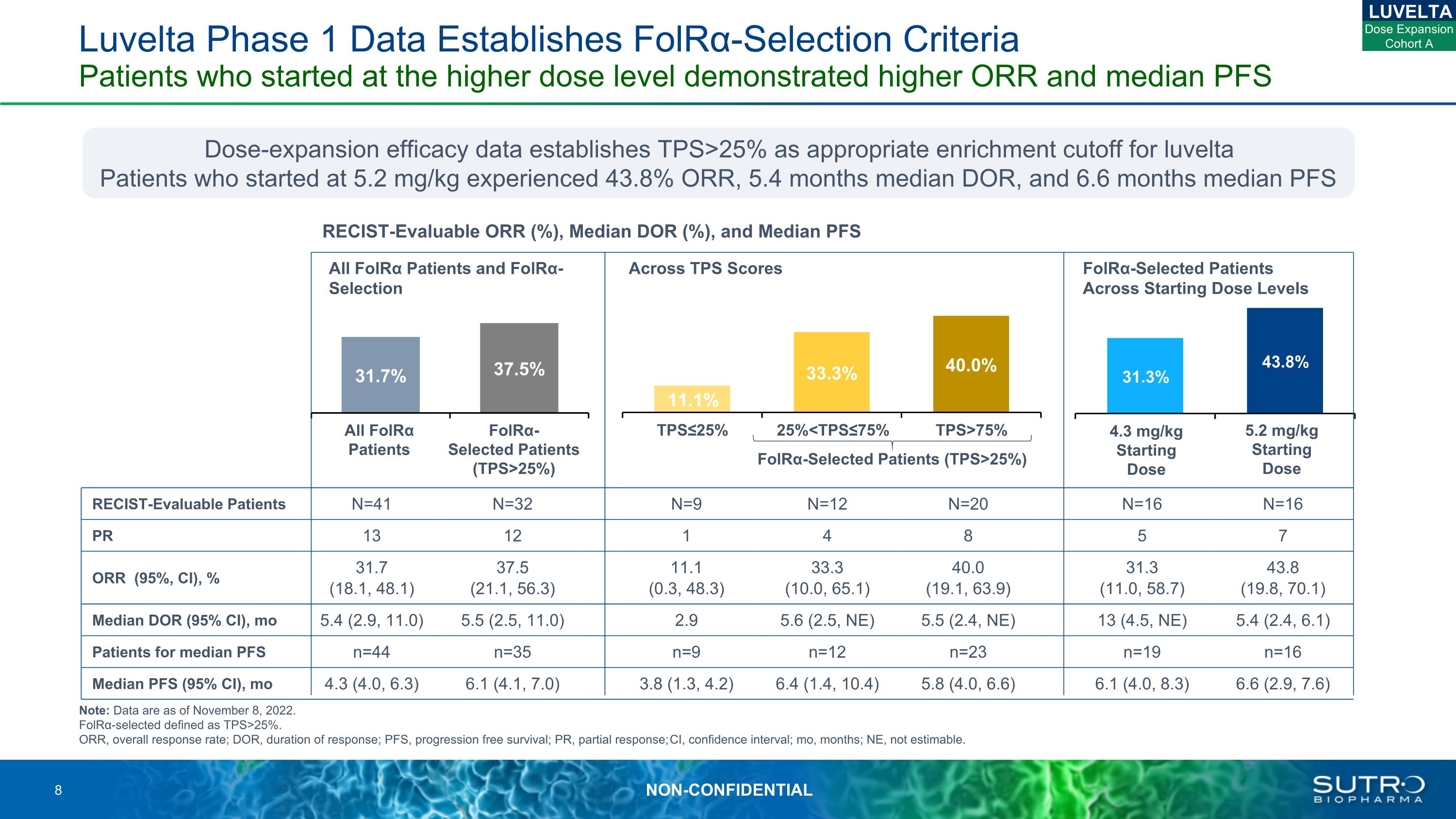
RECIST-Evaluable Patients N=41 N=32 N=9 N=12 N=20 N=16 N=16 PR 13 12 1 4 8 5 7 ORR (95%, CI), % 31.7 (18.1, 48.1) 37.5(21.1, 56.3) 11.1 (0.3, 48.3) 33.3(10.0, 65.1) 40.0(19.1, 63.9) 31.3(11.0, 58.7) 43.8 (19.8, 70.1) Median DOR (95% CI), mo 5.4 (2.9, 11.0) 5.5 (2.5, 11.0) 2.9 5.6 (2.5, NE) 5.5 (2.4, NE) 13 (4.5, NE) 5.4 (2.4, 6.1) Patients for median PFS n=44 n=35 n=9 n=12 n=23 n=19 n=16 Median PFS (95% CI), mo 4.3 (4.0, 6.3) 6.1 (4.1, 7.0) 3.8 (1.3, 4.2) 6.4 (1.4, 10.4) 5.8 (4.0, 6.6) 6.1 (4.0, 8.3) 6.6 (2.9, 7.6) Luvelta Phase 1 Data Establishes FolRα-Selection Criteria Patients who started at the higher dose level demonstrated higher ORR and median PFS Note: Data are as of November 8, 2022. FolRα-selected defined as TPS>25%. ORR, overall response rate; DOR, duration of response; PFS, progression free survival; PR, partial response; CI, confidence interval; mo, months; NE, not estimable. RECIST-Evaluable ORR (%), Median DOR (%), and Median PFS All FolRα Patients and FolRα-Selection Across TPS Scores FolRα-Selected Patients Across Starting Dose Levels All FolRα Patients FolRα-Selected Patients (TPS>25%) 4.3 mg/kg Starting Dose 5.2 mg/kg Starting Dose 25%<TPS≤75% TPS>75% TPS≤25% LUVELTA Dose Expansion Cohort A FolRα-Selected Patients (TPS>25%) Dose-expansion efficacy data establishes TPS>25% as appropriate enrichment cutoff for luveltaPatients who started at 5.2 mg/kg experienced 43.8% ORR, 5.4 months median DOR, and 6.6 months median PFS
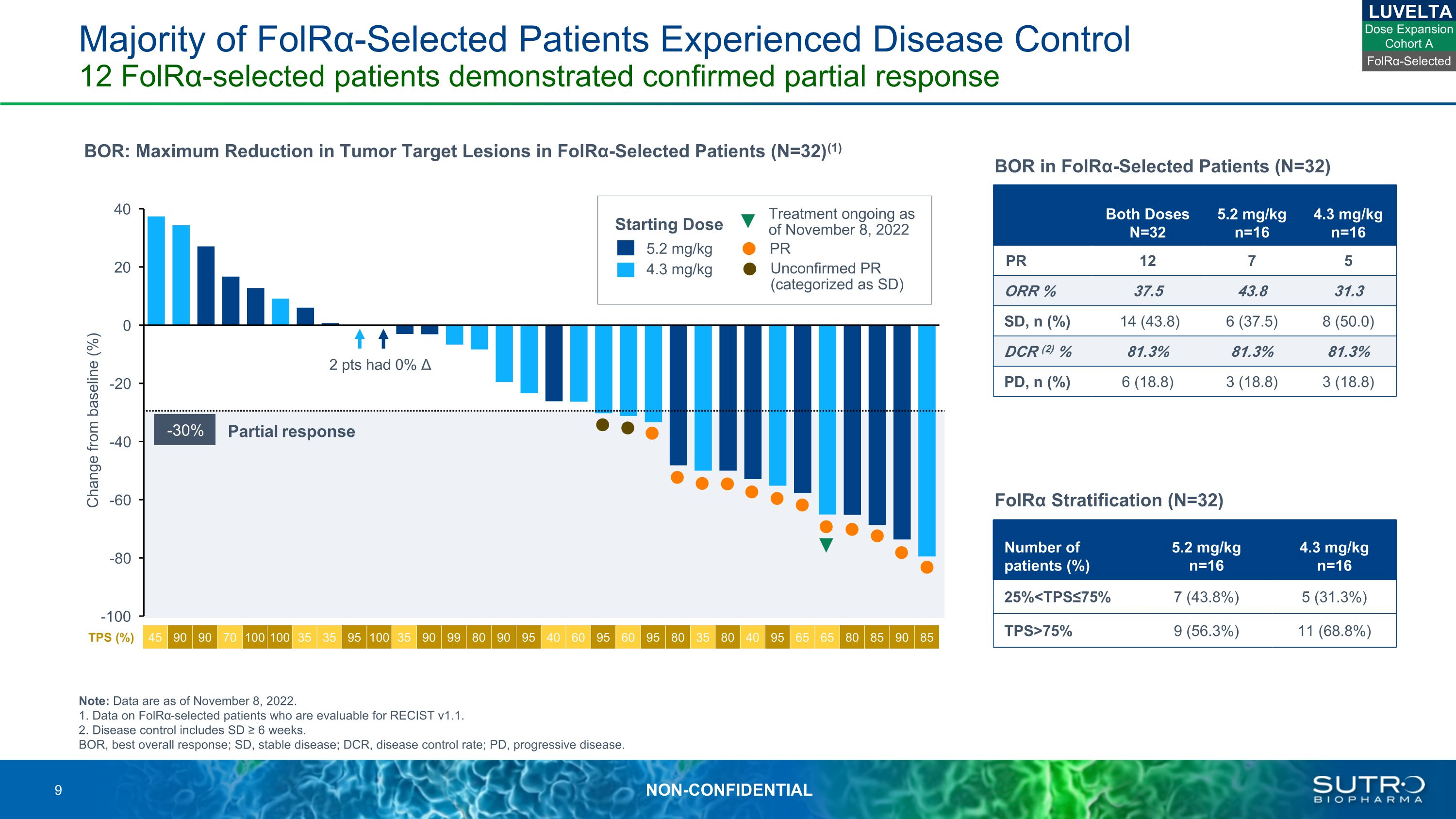
Majority of FolRα-Selected Patients Experienced Disease Control 12 FolRα-selected patients demonstrated confirmed partial response Note: Data are as of November 8, 2022.1. Data on FolRα-selected patients who are evaluable for RECIST v1.1.2. Disease control includes SD ≥ 6 weeks.BOR, best overall response; SD, stable disease; DCR, disease control rate; PD, progressive disease. TPS (%) 45 90 90 70 100 100 35 35 95 100 35 90 99 80 90 95 40 60 95 60 95 80 35 80 40 95 65 65 80 85 90 85 Partial response -30% 2 pts had 0% Δ LUVELTA FolRα-Selected Both Doses N=32 5.2 mg/kg n=16 4.3 mg/kg n=16 PR 12 7 5 ORR % 37.5 43.8 31.3 SD, n (%) 14 (43.8) 6 (37.5) 8 (50.0) DCR (2) % 81.3% 81.3% 81.3% PD, n (%) 6 (18.8) 3 (18.8) 3 (18.8) BOR in FolRα-Selected Patients (N=32) Change from baseline (%) BOR: Maximum Reduction in Tumor Target Lesions in FolRα-Selected Patients (N=32)(1) 5.2 mg/kg 4.3 mg/kg Starting Dose PR Unconfirmed PR (categorized as SD) Treatment ongoing as of November 8, 2022 Number of patients (%) 5.2 mg/kg n=16 4.3 mg/kg n=16 25%<TPS≤75% 7 (43.8%) 5 (31.3%) TPS>75% 9 (56.3%) 11 (68.8%) FolRα Stratification (N=32) Dose Expansion Cohort A
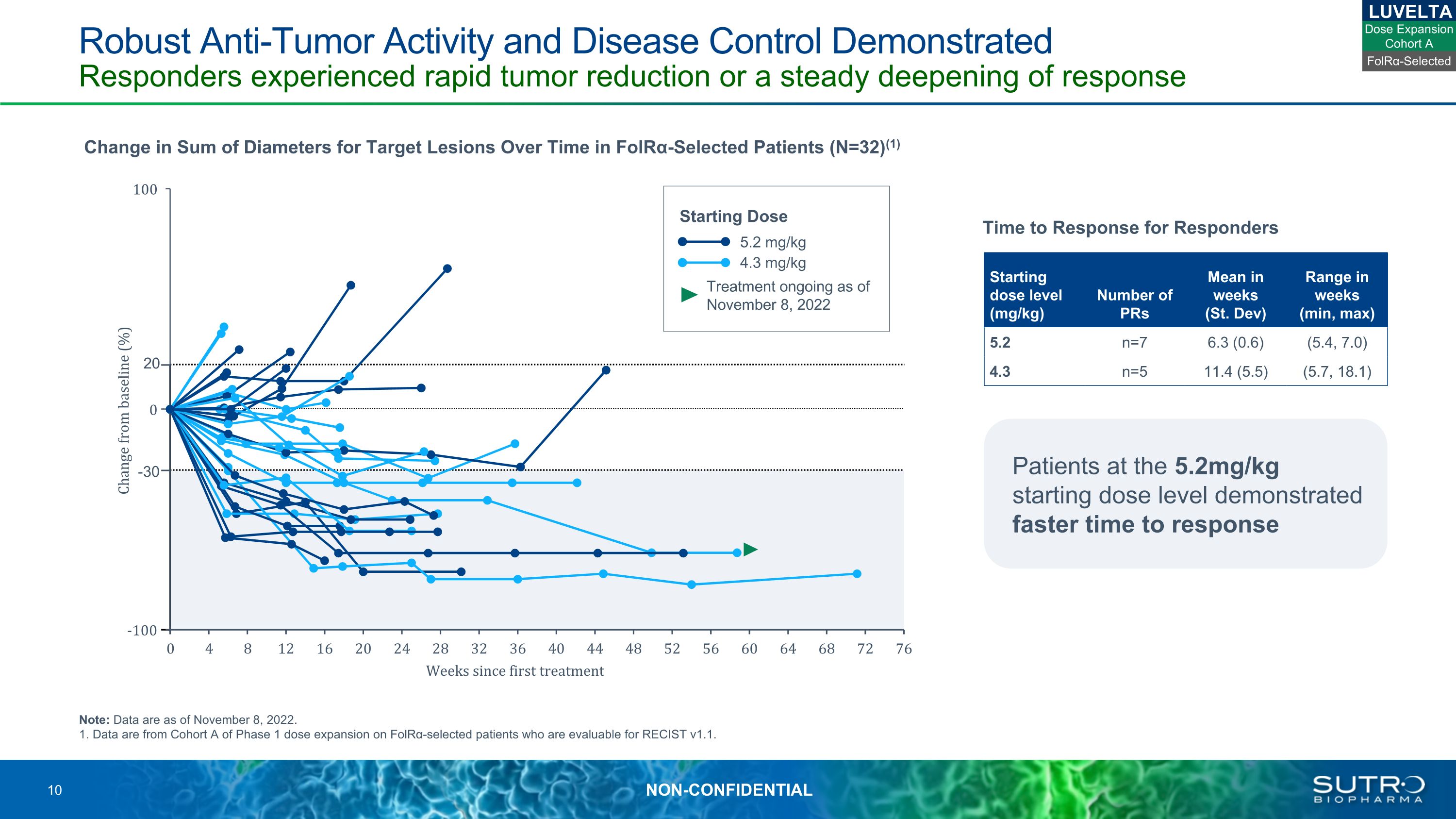
Robust Anti-Tumor Activity and Disease Control Demonstrated Responders experienced rapid tumor reduction or a steady deepening of response Note: Data are as of November 8, 2022.1. Data are from Cohort A of Phase 1 dose expansion on FolRα-selected patients who are evaluable for RECIST v1.1. 20 -30 Change in Sum of Diameters for Target Lesions Over Time in FolRα-Selected Patients (N=32)(1) LUVELTA Starting dose level (mg/kg) Number of PRs Mean in weeks(St. Dev) Range in weeks(min, max) 5.2 n=7 6.3 (0.6) (5.4, 7.0) 4.3 n=5 11.4 (5.5) (5.7, 18.1) Time to Response for Responders 5.2 mg/kg 4.3 mg/kg Treatment ongoing as of November 8, 2022 Starting Dose Patients at the 5.2mg/kg starting dose level demonstrated faster time to response FolRα-Selected Dose Expansion Cohort A
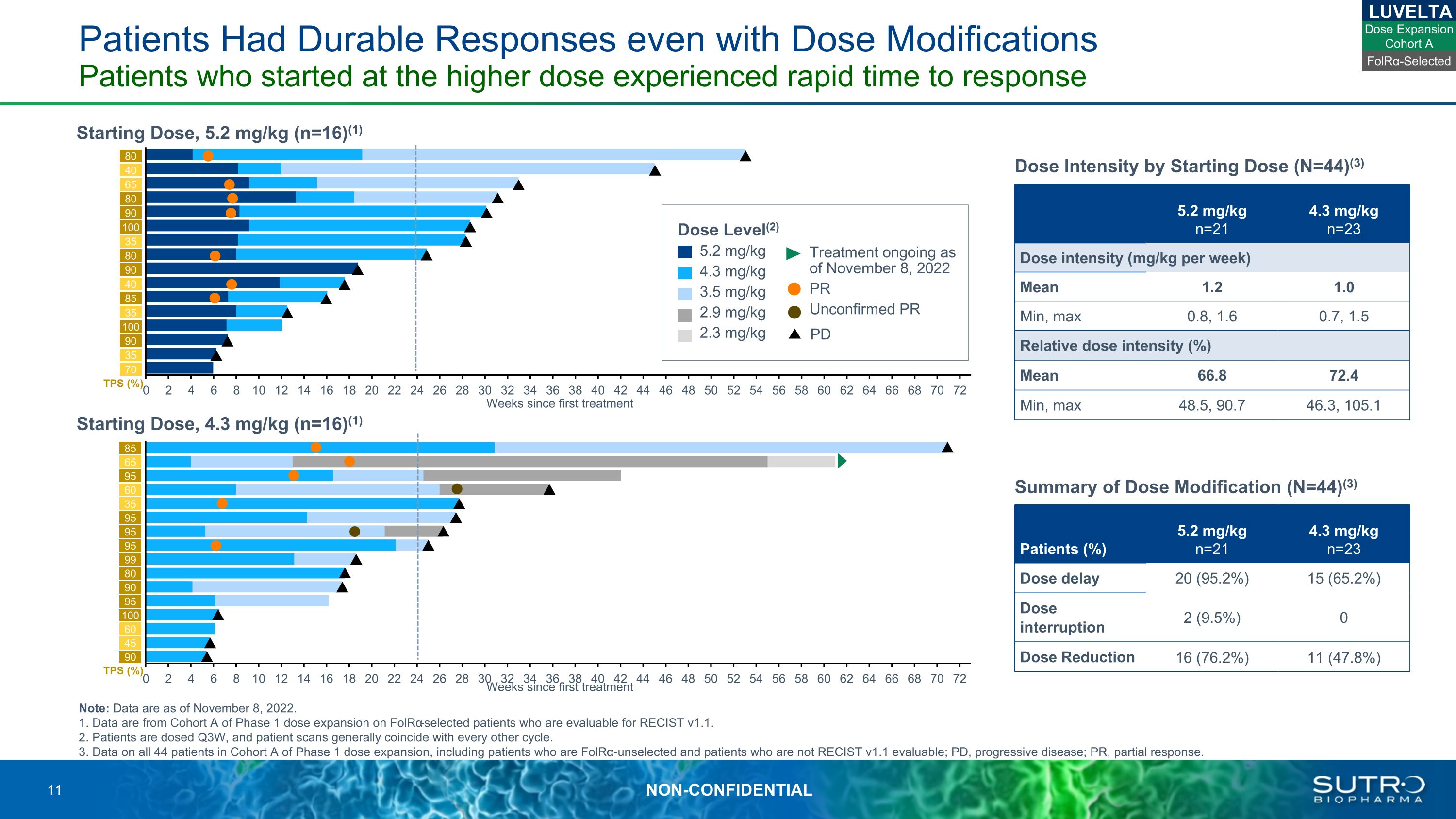
Patients Had Durable Responses even with Dose Modifications Patients who started at the higher dose experienced rapid time to response Note: Data are as of November 8, 2022. 1. Data are from Cohort A of Phase 1 dose expansion on FolRα-selected patients who are evaluable for RECIST v1.1.2. Patients are dosed Q3W, and patient scans generally coincide with every other cycle.3. Data on all 44 patients in Cohort A of Phase 1 dose expansion, including patients who are FolRα-unselected and patients who are not RECIST v1.1 evaluable; PD, progressive disease; PR, partial response. Starting Dose, 5.2 mg/kg (n=16)(1) 80 40 65 80 90 80 90 40 85 35 100 90 35 35 100 LUVELTA 5.2 mg/kg n=21 4.3 mg/kg n=23 N=23 Dose intensity (mg/kg per week) Mean 1.2 1.0 Min, max 0.8, 1.6 0.7, 1.5 Relative dose intensity (%) Mean 66.8 72.4 Min, max 48.5, 90.7 46.3, 105.1 Dose Intensity by Starting Dose (N=44)(3) 85 65 95 60 35 95 95 95 99 80 90 95 100 60 45 90 TPS (%) 70 Dose Level(2) 5.2 mg/kg 4.3 mg/kg 3.5 mg/kg 2.9 mg/kg 2.3 mg/kg Treatment ongoing as of November 8, 2022 PR Unconfirmed PR PD Weeks since first treatment Starting Dose, 4.3 mg/kg (n=16)(1) TPS (%) Weeks since first treatment FolRα-Selected Dose Expansion Cohort A 5.2 mg/kg n=21 4.3 mg/kg n=23 Patients (%) N=23 Dose delay 20 (95.2%) 15 (65.2%) Dose interruption 2 (9.5%) 0 Dose Reduction 16 (76.2%) 11 (47.8%) Summary of Dose Modification (N=44)(3)
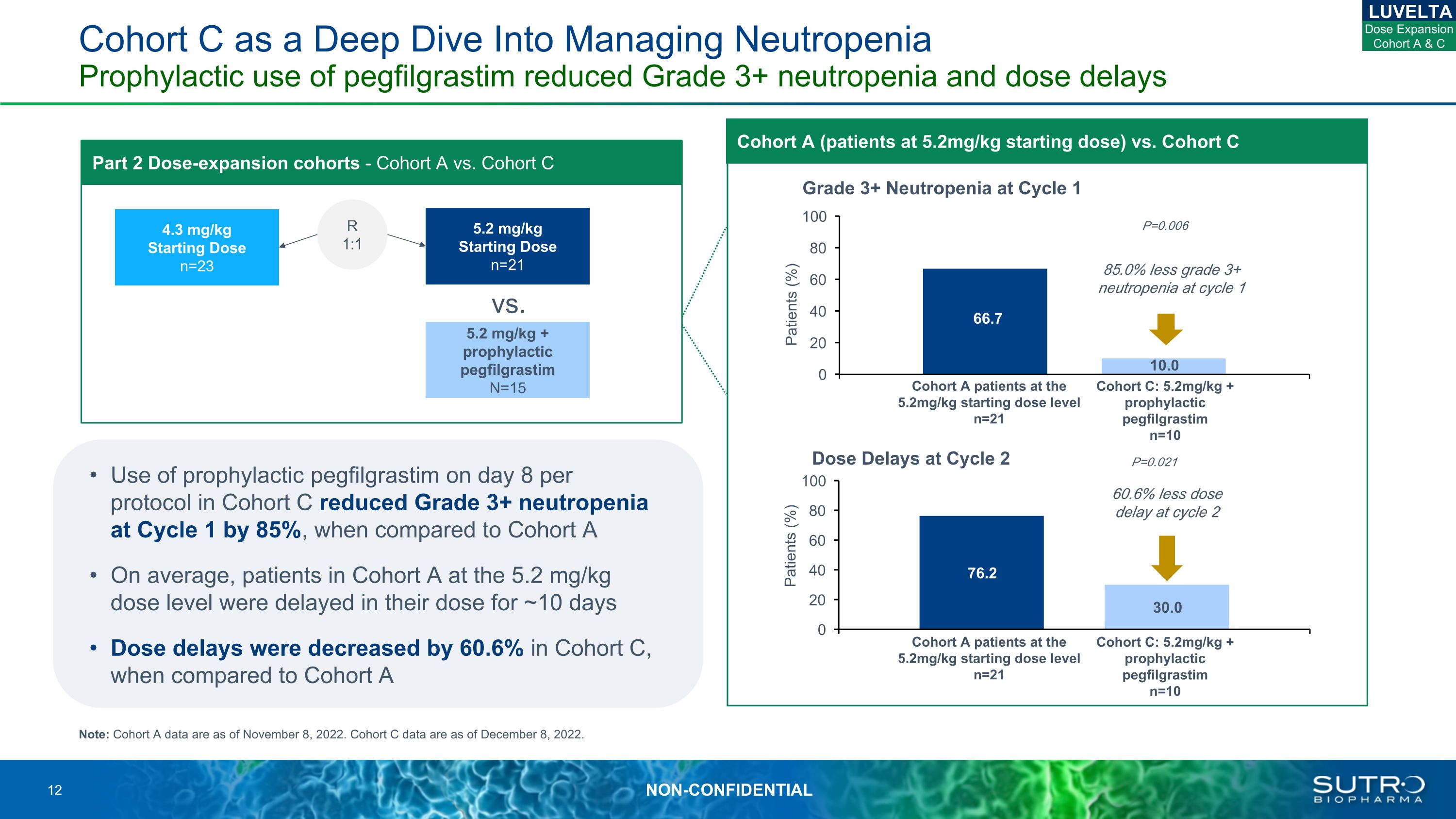
Cohort C as a Deep Dive Into Managing Neutropenia Prophylactic use of pegfilgrastim reduced Grade 3+ neutropenia and dose delays Grade 3+ Neutropenia at Cycle 1 P=0.006 85.0% less grade 3+ neutropenia at cycle 1 Dose Delays at Cycle 2 P=0.021 60.6% less dose delay at cycle 2 Part 2 Dose-expansion cohorts - Cohort A vs. Cohort C R 1:1 Note: Cohort A data are as of November 8, 2022. Cohort C data are as of December 8, 2022. LUVELTA Cohort A patients at the 5.2mg/kg starting dose level n=21 Cohort C: 5.2mg/kg + prophylactic pegfilgrastim n=10 4.3 mg/kg Starting Dose n=23 5.2 mg/kg Starting Dose n=21 5.2 mg/kg + prophylactic pegfilgrastim N=15 vs. Cohort A patients at the 5.2mg/kg starting dose level n=21 Cohort C: 5.2mg/kg + prophylactic pegfilgrastim n=10 Use of prophylactic pegfilgrastim on day 8 per protocol in Cohort C reduced Grade 3+ neutropenia at Cycle 1 by 85%, when compared to Cohort A On average, patients in Cohort A at the 5.2 mg/kg dose level were delayed in their dose for ~10 days Dose delays were decreased by 60.6% in Cohort C, when compared to Cohort A Dose Expansion Cohort A & C Cohort A (patients at 5.2mg/kg starting dose) vs. Cohort C
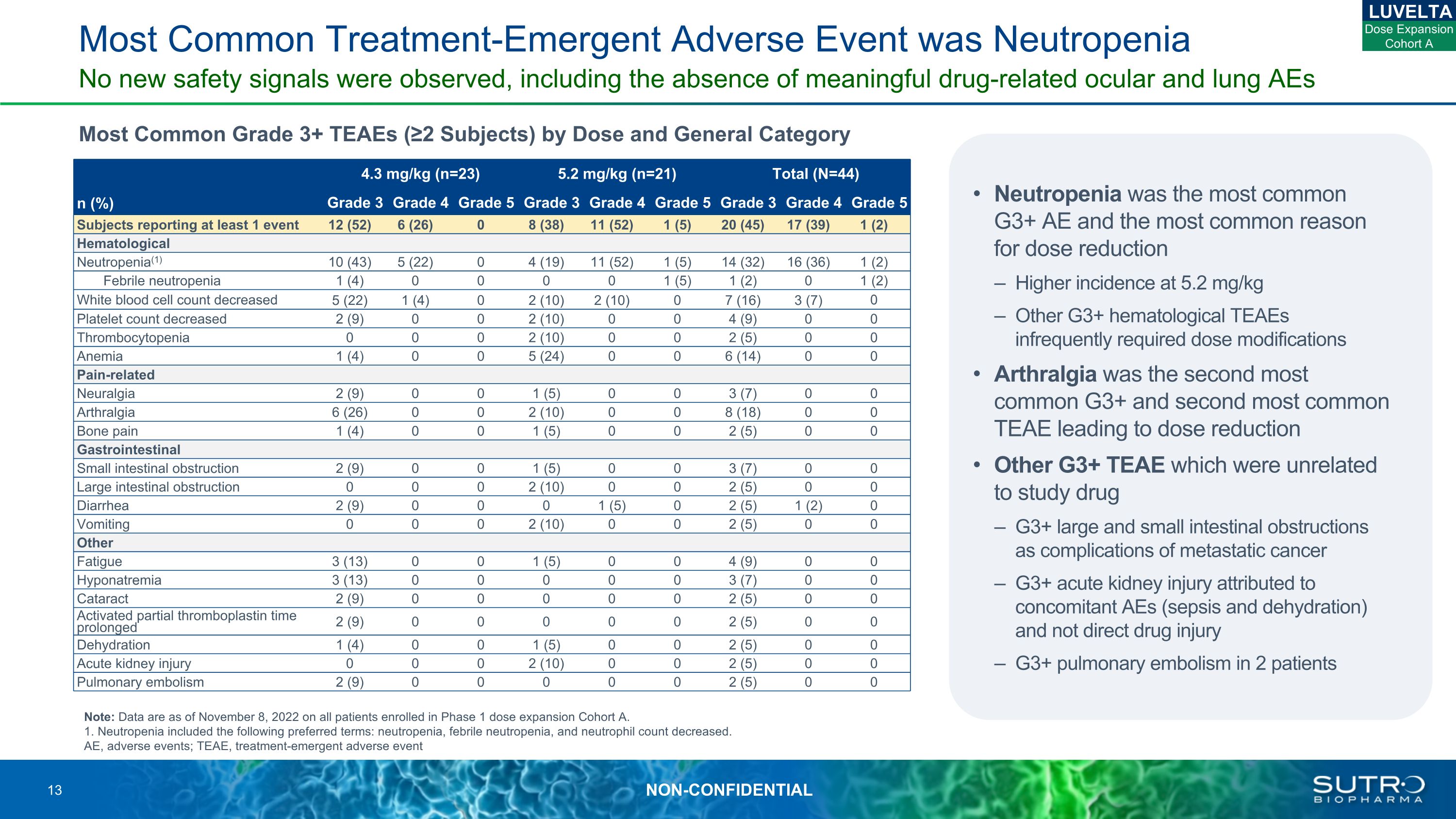
Most Common Treatment-Emergent Adverse Event was Neutropenia Most Common Grade 3+ TEAEs (≥2 Subjects) by Dose and General Category No new safety signals were observed, including the absence of meaningful drug-related ocular and lung AEs Note: Data are as of November 8, 2022 on all patients enrolled in Phase 1 dose expansion Cohort A. 1. Neutropenia included the following preferred terms: neutropenia, febrile neutropenia, and neutrophil count decreased. AE, adverse events; TEAE, treatment-emergent adverse event 4.3 mg/kg (n=23) 5.2 mg/kg (n=21) Total (N=44) n (%) Grade 3 Grade 4 Grade 5 Grade 3 Grade 4 Grade 5 Grade 3 Grade 4 Grade 5 Subjects reporting at least 1 event 12 (52) 6 (26) 0 8 (38) 11 (52) 1 (5) 20 (45) 17 (39) 1 (2) Hematological Neutropenia(1) 10 (43) 5 (22) 0 4 (19) 11 (52) 1 (5) 14 (32) 16 (36) 1 (2) Febrile neutropenia 1 (4) 0 0 0 0 1 (5) 1 (2) 0 1 (2) White blood cell count decreased 5 (22) 1 (4) 0 2 (10) 2 (10) 0 7 (16) 3 (7) 0 Platelet count decreased 2 (9) 0 0 2 (10) 0 0 4 (9) 0 0 Thrombocytopenia 0 0 0 2 (10) 0 0 2 (5) 0 0 Anemia 1 (4) 0 0 5 (24) 0 0 6 (14) 0 0 Pain-related Neuralgia 2 (9) 0 0 1 (5) 0 0 3 (7) 0 0 Arthralgia 6 (26) 0 0 2 (10) 0 0 8 (18) 0 0 Bone pain 1 (4) 0 0 1 (5) 0 0 2 (5) 0 0 Gastrointestinal Small intestinal obstruction 2 (9) 0 0 1 (5) 0 0 3 (7) 0 0 Large intestinal obstruction 0 0 0 2 (10) 0 0 2 (5) 0 0 Diarrhea 2 (9) 0 0 0 1 (5) 0 2 (5) 1 (2) 0 Vomiting 0 0 0 2 (10) 0 0 2 (5) 0 0 Other Fatigue 3 (13) 0 0 1 (5) 0 0 4 (9) 0 0 Hyponatremia 3 (13) 0 0 0 0 0 3 (7) 0 0 Cataract 2 (9) 0 0 0 0 0 2 (5) 0 0 Activated partial thromboplastin time prolonged 2 (9) 0 0 0 0 0 2 (5) 0 0 Dehydration 1 (4) 0 0 1 (5) 0 0 2 (5) 0 0 Acute kidney injury 0 0 0 2 (10) 0 0 2 (5) 0 0 Pulmonary embolism 2 (9) 0 0 0 0 0 2 (5) 0 0 Neutropenia was the most common G3+ AE and the most common reason for dose reduction Higher incidence at 5.2 mg/kg Other G3+ hematological TEAEs infrequently required dose modifications Arthralgia was the second most common G3+ and second most common TEAE leading to dose reduction Other G3+ TEAE which were unrelated to study drug G3+ large and small intestinal obstructions as complications of metastatic cancer G3+ acute kidney injury attributed to concomitant AEs (sepsis and dehydration) and not direct drug injury G3+ pulmonary embolism in 2 patients LUVELTA Dose Expansion Cohort A

Registrational Path Forward for Luveltamab Tazevibulin (Luvelta, STRO-002)
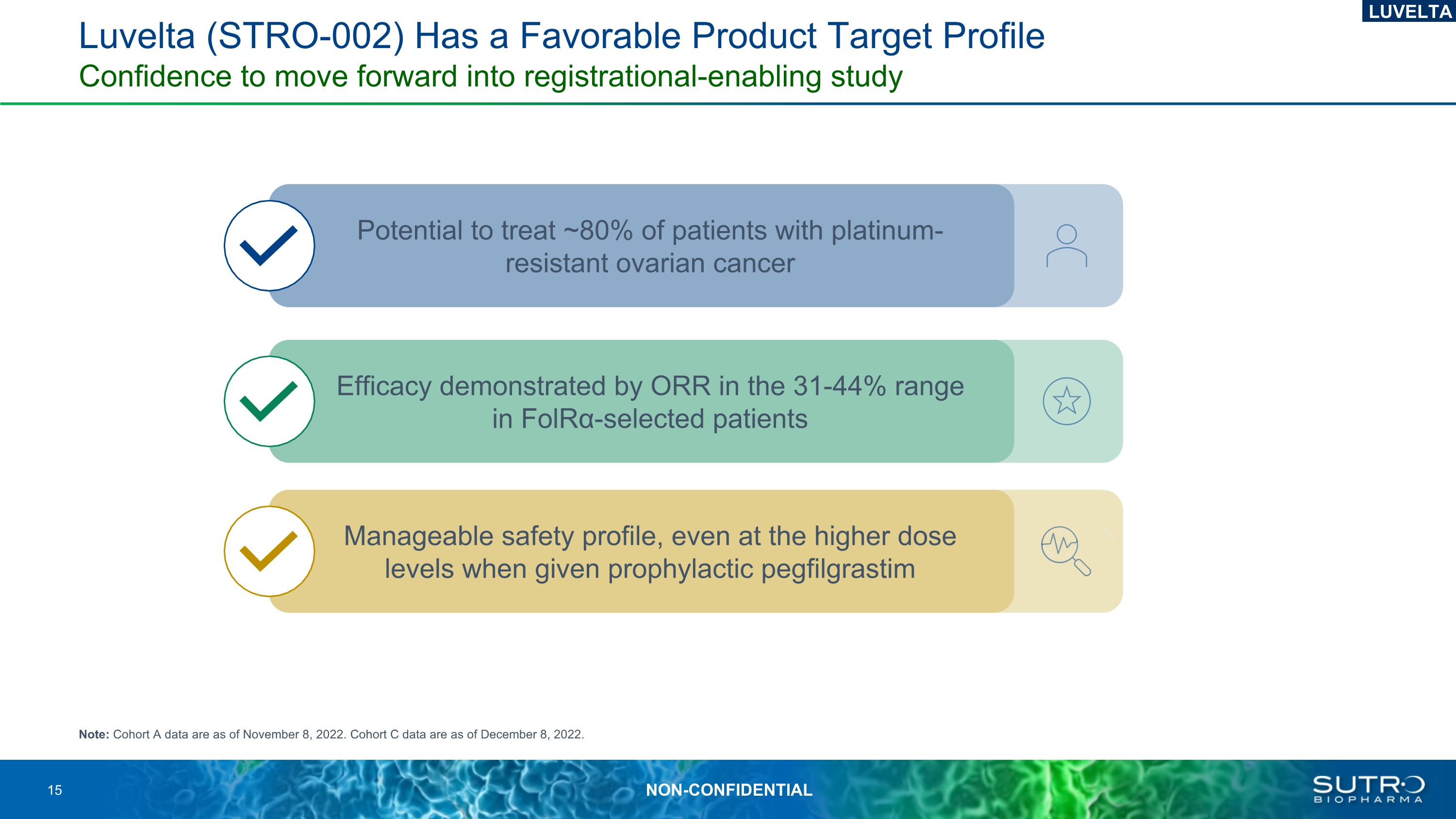
Luvelta (STRO-002) Has a Favorable Product Target Profile Confidence to move forward into registrational-enabling study Note: Cohort A data are as of November 8, 2022. Cohort C data are as of December 8, 2022. Potential to treat ~80% of patients with platinum-resistant ovarian cancer Efficacy demonstrated by ORR in the 31-44% range in FolRα-selected patients Manageable safety profile, even at the higher dose levels when given prophylactic pegfilgrastim LUVELTA
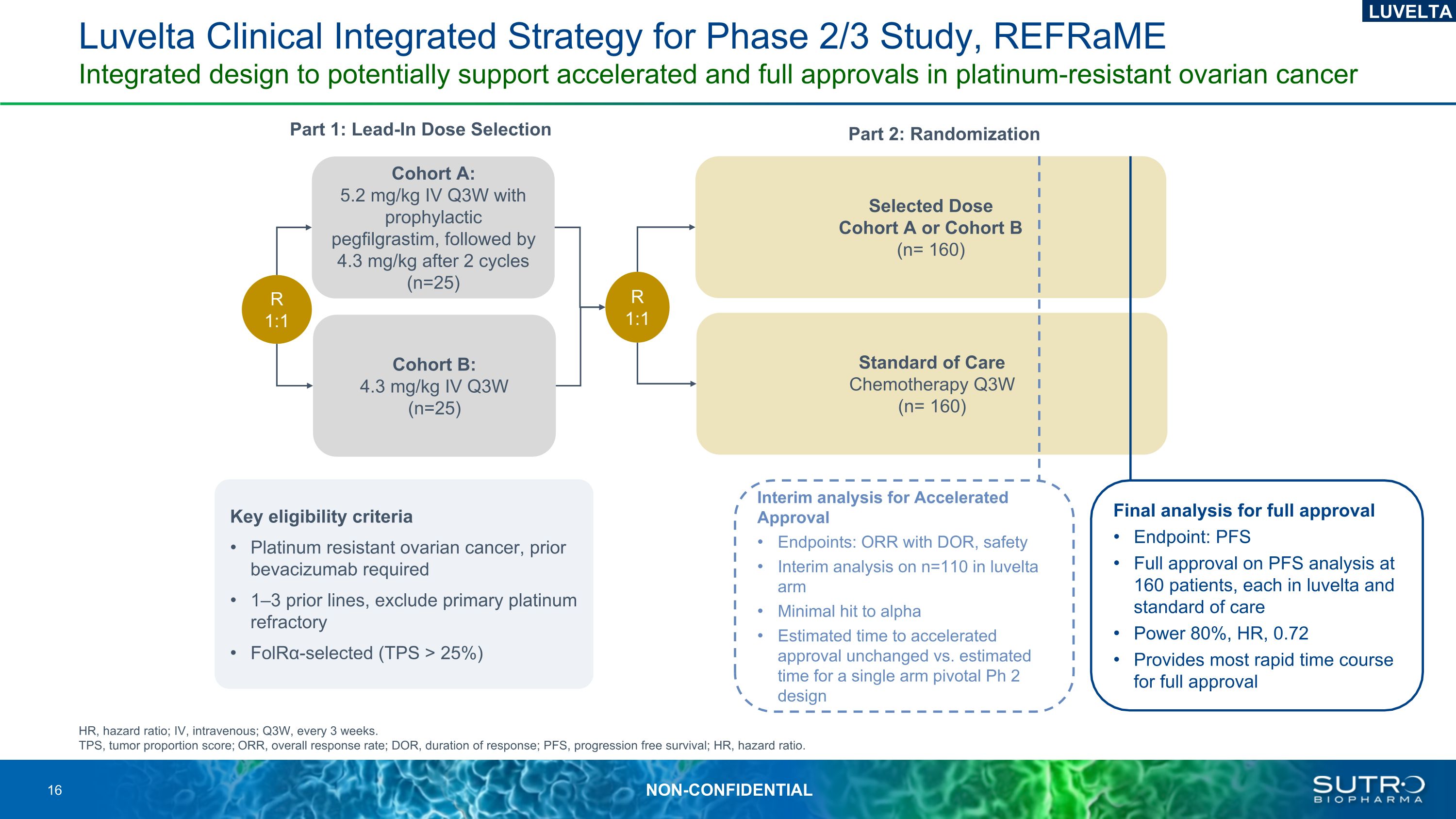
Luvelta Clinical Integrated Strategy for Phase 2/3 Study, REFRaMEIntegrated design to potentially support accelerated and full approvals in platinum-resistant ovarian cancer Part 1: Lead-In Dose Selection Key eligibility criteria Platinum resistant ovarian cancer, prior bevacizumab required 1–3 prior lines, exclude primary platinum refractory FolRα-selected (TPS > 25%) Part 2: Randomization LUVELTA Cohort A: 5.2 mg/kg IV Q3W with prophylactic pegfilgrastim, followed by 4.3 mg/kg after 2 cycles (n=25) Cohort B: 4.3 mg/kg IV Q3W (n=25) Interim analysis for Accelerated Approval Endpoints: ORR with DOR, safety Interim analysis on n=110 in luvelta arm Minimal hit to alpha Estimated time to accelerated approval unchanged vs. estimated time for a single arm pivotal Ph 2 design Standard of Care Chemotherapy Q3W (n= 160) R 1:1 R 1:1 Selected Dose Cohort A or Cohort B(n= 160) Final analysis for full approval Endpoint: PFS Full approval on PFS analysis at 160 patients, each in luvelta and standard of care Power 80%, HR, 0.72 Provides most rapid time course for full approval HR, hazard ratio; IV, intravenous; Q3W, every 3 weeks.TPS, tumor proportion score; ORR, overall response rate; DOR, duration of response; PFS, progression free survival; HR, hazard ratio.

Market Opportunity for Luveltamab Tazevibulin (Luvelta, STRO-002) and Ovarian Cancer
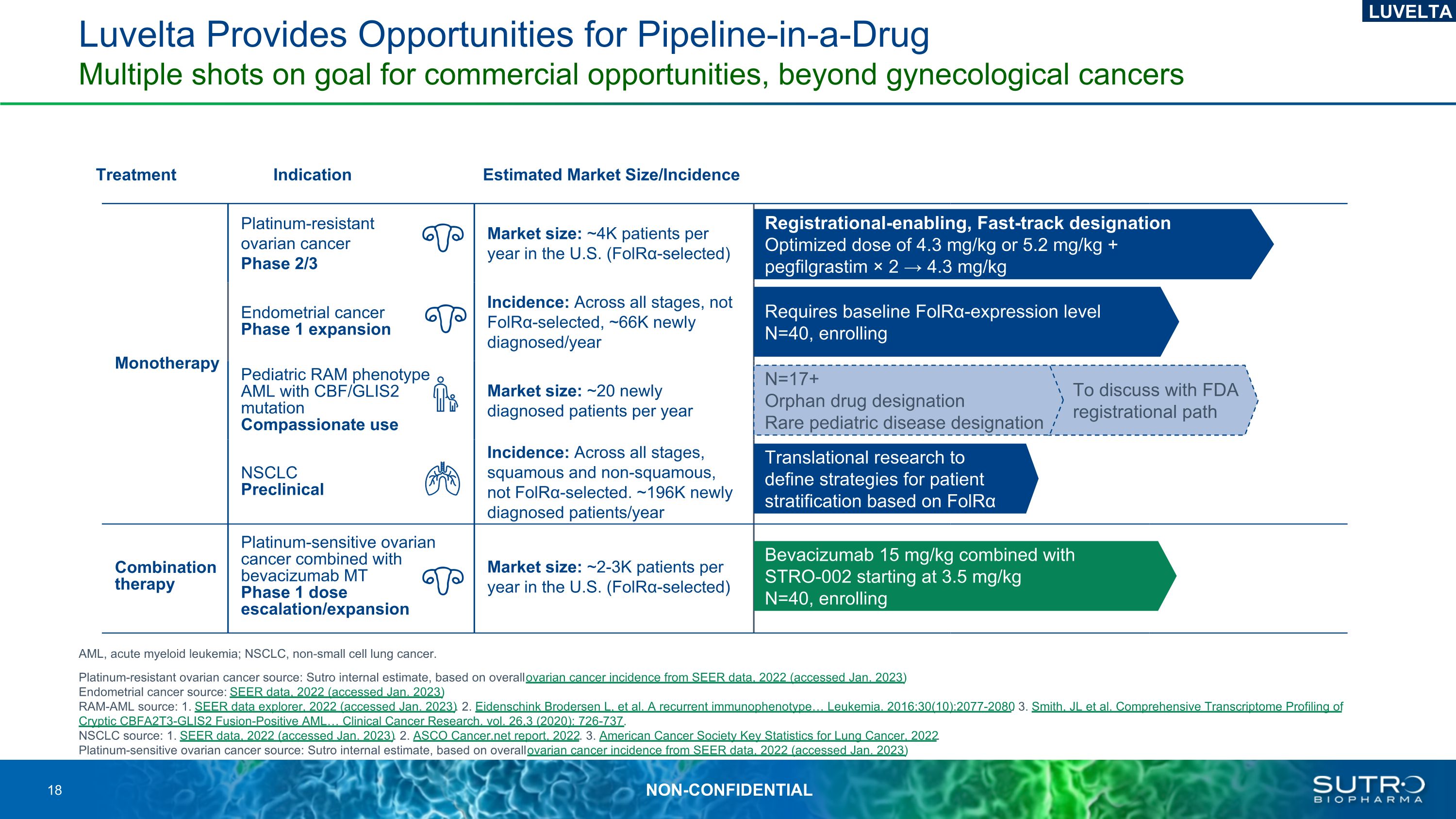
Monotherapy Platinum-resistant ovarian cancer Phase 2/3 Market size: ~4K patients per year in the U.S. (FolRα-selected) Endometrial cancer Phase 1 expansion Incidence: Across all stages, not FolRα-selected, ~66K newly diagnosed/year Pediatric RAM phenotype AML with CBF/GLIS2 mutationCompassionate use Market size: ~20 newly diagnosed patients per year NSCLC Preclinical Incidence: Across all stages, squamous and non-squamous, not FolRα-selected. ~196K newly diagnosed patients/year Combination therapy Platinum-sensitive ovarian cancer combined with bevacizumab MT Phase 1 dose escalation/expansion Market size: ~2-3K patients per year in the U.S. (FolRα-selected) Bevacizumab 15 mg/kg combined with STRO-002 starting at 3.5 mg/kg N=40, enrolling Translational research to define strategies for patient stratification based on FolRα Requires baseline FolRα-expression level N=40, enrolling Registrational-enabling, Fast-track designation Optimized dose of 4.3 mg/kg or 5.2 mg/kg + pegfilgrastim × 2 → 4.3 mg/kg Treatment Indication Estimated Market Size/Incidence To discuss with FDA registrational path N=17+Orphan drug designation Rare pediatric disease designation Luvelta Provides Opportunities for Pipeline-in-a-DrugMultiple shots on goal for commercial opportunities, beyond gynecological cancers LUVELTA AML, acute myeloid leukemia; NSCLC, non-small cell lung cancer. Platinum-resistant ovarian cancer source: Sutro internal estimate, based on overall ovarian cancer incidence from SEER data, 2022 (accessed Jan. 2023)Endometrial cancer source: SEER data, 2022 (accessed Jan. 2023)RAM-AML source: 1. SEER data explorer, 2022 (accessed Jan. 2023). 2. Eidenschink Brodersen L, et al. A recurrent immunophenotype Leukemia. 2016;30(10):2077-2080. 3. Smith, JL et al. Comprehensive Transcriptome Profiling of Cryptic CBFA2T3-GLIS2 Fusion-Positive AML Clinical Cancer Research. vol. 26,3 (2020): 726-737.NSCLC source: 1. SEER data, 2022 (accessed Jan. 2023). 2. ASCO Cancer.net report, 2022. 3. American Cancer Society Key Statistics for Lung Cancer, 2022.Platinum-sensitive ovarian cancer source: Sutro internal estimate, based on overall ovarian cancer incidence from SEER data, 2022 (accessed Jan. 2023)
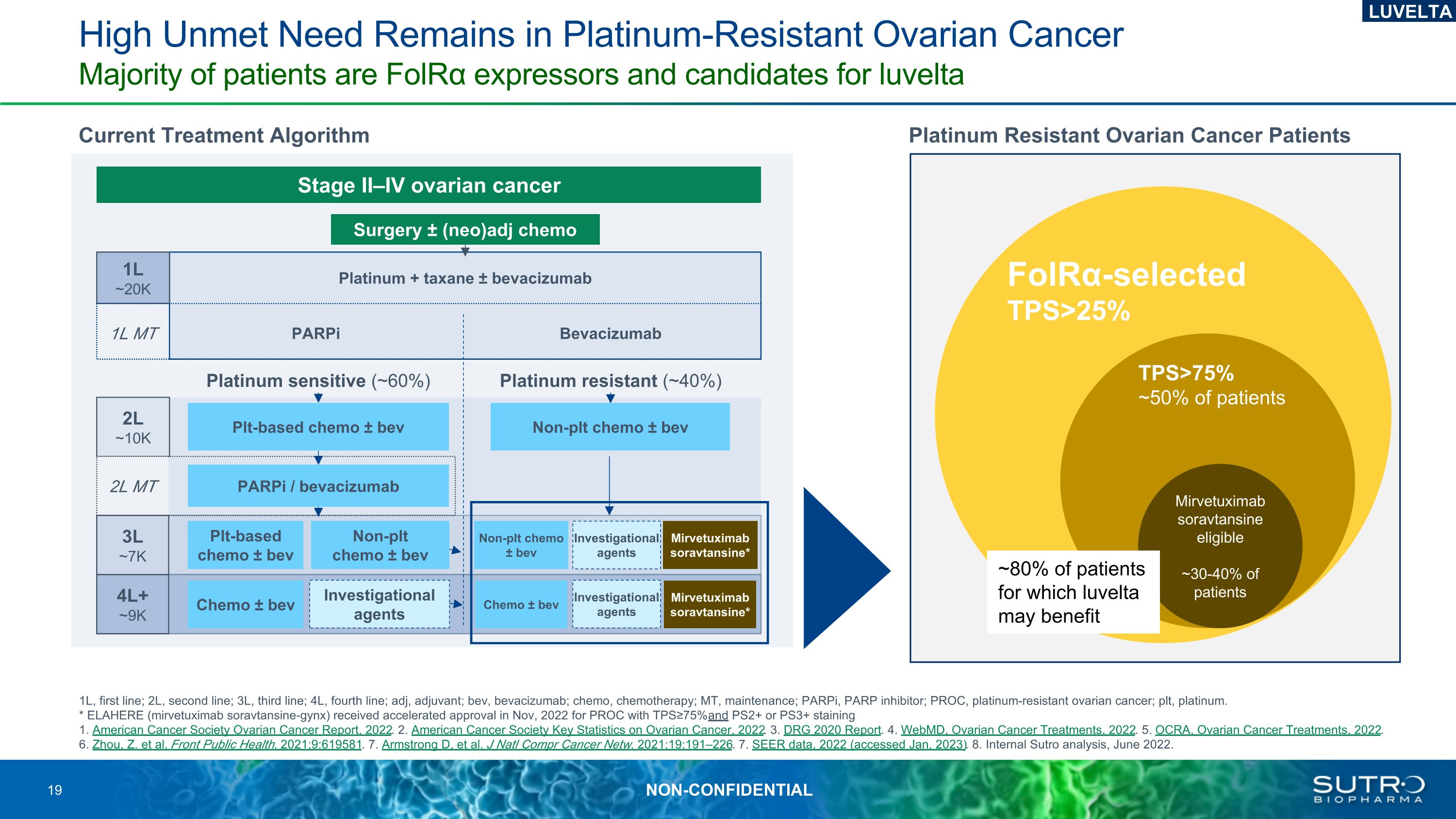
High Unmet Need Remains in Platinum-Resistant Ovarian CancerMajority of patients are FolRα expressors and candidates for luvelta 1L, first line; 2L, second line; 3L, third line; 4L, fourth line; adj, adjuvant; bev, bevacizumab; chemo, chemotherapy; MT, maintenance; PARPi, PARP inhibitor; PROC, platinum-resistant ovarian cancer; plt, platinum. * ELAHERE (mirvetuximab soravtansine-gynx) received accelerated approval in Nov, 2022 for PROC with TPS≥75% and PS2+ or PS3+ staining1. American Cancer Society Ovarian Cancer Report, 2022. 2. American Cancer Society Key Statistics on Ovarian Cancer, 2022. 3. DRG 2020 Report. 4. WebMD, Ovarian Cancer Treatments, 2022. 5. OCRA, Ovarian Cancer Treatments, 2022. 6. Zhou, Z. et al. Front Public Health. 2021;9:619581. 7. Armstrong D, et al. J Natl Compr Cancer Netw. 2021;19:191–226. 7. SEER data, 2022 (accessed Jan, 2023). 8. Internal Sutro analysis, June 2022. Current Treatment Algorithm Platinum Resistant Ovarian Cancer Patients FolRα-selected TPS>25% Surgery ± (neo)adj chemo 1L ~20K Platinum + taxane ± bevacizumab PARPi Bevacizumab Platinum sensitive (~60%) Platinum resistant (~40%) 2L ~10K Plt-based chemo ± bev 4L+ ~9K Plt-based chemo ± bev Non-plt chemo ± bev Stage II–IV ovarian cancer 2L MT 1L MT PARPi / bevacizumab 3L ~7K Investigational agents Non-plt chemo ± bev Non-plt chemo ± bev Chemo ± bev Investigational agents Chemo ± bev Investigational agents TPS>75% ~50% of patients LUVELTA Mirvetuximab soravtansine* Mirvetuximab soravtansine* Mirvetuximab soravtansine eligible ~30-40% of patients ~80% of patients for which luvelta may benefit

Closing Remarks and Q&A