EX-99.1
Published on June 16, 2022
Company Overview June 2022 Sutro BiopharmaNASDAQ: STRO Exhibit 99.1

Forward Looking Statements This presentation and the accompanying oral presentation contain “forward-looking” statements that are based on our management’s beliefs and assumptions and on information currently available to management. Forward-looking statements include all statements other than statements of historical fact contained in this presentation, including information concerning our future financial performance, business plans and objectives, current and future clinical activities, timing, design and success of our ongoing and planned clinical trials and related data, updates and results of our clinical trials and related data, timing and success of our planned development activities, our ability to obtain and maintain regulatory approval, the potential therapeutic benefits and economic value of our product candidates, potential growth opportunities, financing plans, potential future milestone and royalty payments, competitive position, industry environment and potential market opportunities for the Company’s product candidates. Forward-looking statements are subject to known and unknown risks, uncertainties, assumptions and other factors, including risks and uncertainties related to our cash forecasts, our and our collaborators’ ability to advance our product candidates, the receipt, feedback and timing of potential regulatory submissions, designations, approvals and commercialization of product candidates, the design, timing and results of preclinical and clinical trials, and the expected impact of the COVID-19 pandemic on our operations. It is not possible for our management to predict all risks, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements we may make. These factors, together with those that may be described in greater detail under the heading “Risk Factors” contained in our most recent Annual Report on Form 10-K, Quarterly Report on Form 10-Q and other reports the company files from time to time with the Securities and Exchange Commission, may cause our actual results, performance or achievements to differ materially and adversely from those anticipated or implied by our forward-looking statements. You should not rely upon forward-looking statements as predictions of future events. Although our management believes that the expectations reflected in our forward-looking statements are reasonable, we cannot guarantee that the future results, levels of activity, performance or events and circumstances described in the forward-looking statements will be achieved or occur. Moreover, neither we nor our management assume responsibility for the accuracy and completeness of the forward-looking statements. We undertake no obligation to publicly update any forward-looking statements for any reason after the date of this presentation to conform these statements to actual results or to changes in our expectations, except as required by law. This presentation also contains estimates and other statistical data made by independent parties and by us relating to market size and growth and other data about our industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. In addition, projections, assumptions, and estimates of our future performance and the future performance of the markets in which we operate are necessarily subject to a high degree of uncertainty and risk.
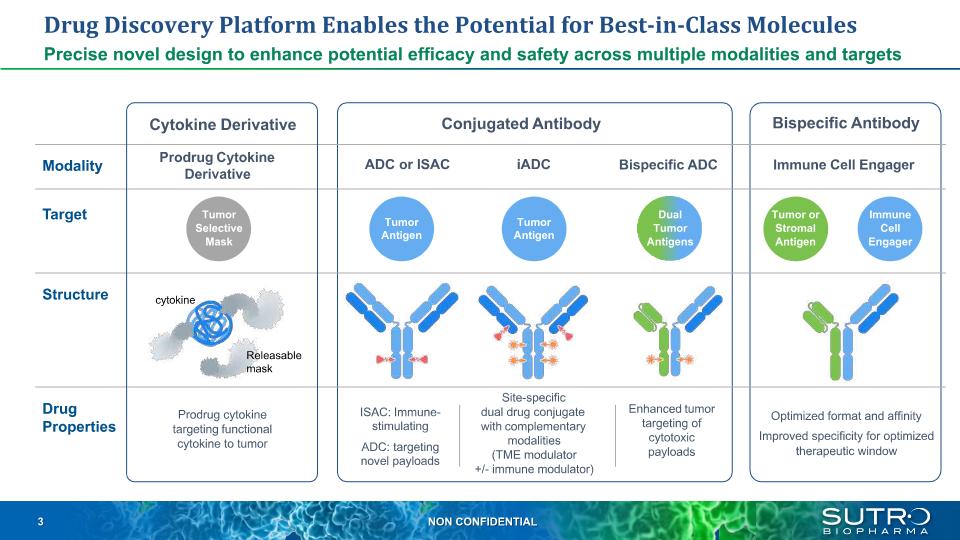
Drug Discovery Platform Enables the Potential for Best-in-Class Molecules Precise novel design to enhance potential efficacy and safety across multiple modalities and targets Enhanced tumor targeting of cytotoxic payloads Bispecific ADC ISAC: Immune- stimulating ADC: targeting novel payloads Bispecific Antibody Tumor or Stromal Antigen Immune Cell Engager Optimized format and affinity Improved specificity for optimized therapeutic window Tumor Antigen Dual Tumor Antigens Conjugated Antibody ADC or ISAC iADC Tumor Antigen Site-specific dual drug conjugate with complementary modalities (TME modulator +/- immune modulator) Immune Cell Engager Modality Drug Properties Target Structure Cytokine Derivative Prodrug cytokine targeting functional cytokine to tumor Prodrug Cytokine Derivative Tumor Selective Mask Releasable mask cytokine
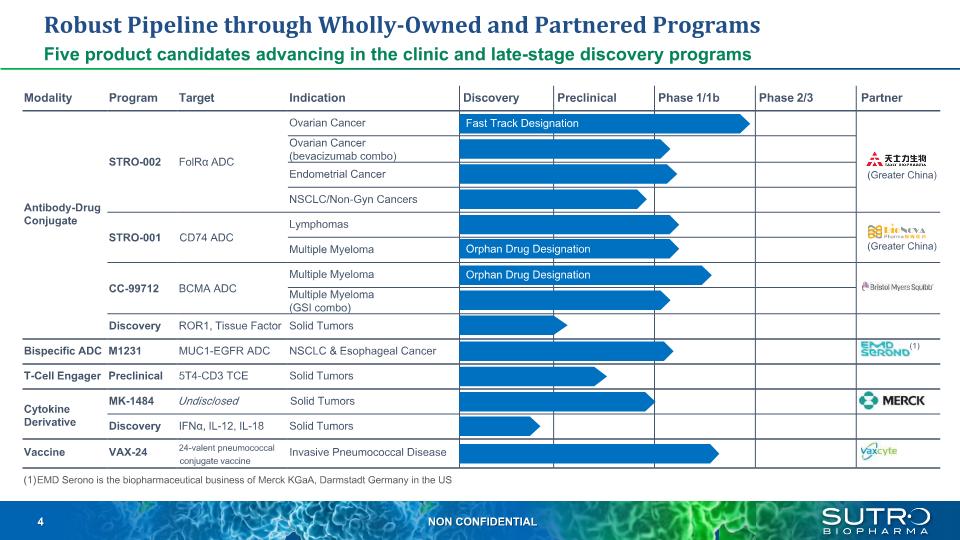
Robust Pipeline through Wholly-Owned and Partnered Programs Five product candidates advancing in the clinic and late-stage discovery programs Modality Program Target Indication Discovery Preclinical Phase 1/1b Phase 2/3 Partner Antibody-Drug Conjugate STRO-002 FolRα ADC Ovarian Cancer Ovarian Cancer (bevacizumab combo) Endometrial Cancer NSCLC/Non-Gyn Cancers STRO-001 CD74 ADC Lymphomas Multiple Myeloma CC-99712 BCMA ADC Multiple Myeloma Multiple Myeloma (GSI combo) Discovery ROR1, Tissue Factor Solid Tumors Bispecific ADC M1231 MUC1-EGFR ADC NSCLC & Esophageal Cancer T-Cell Engager Preclinical 5T4-CD3 TCE Solid Tumors Cytokine Derivative MK-1484 Undisclosed Solid Tumors Discovery IFNα, IL-12, IL-18 Solid Tumors Vaccine VAX-24 24-valent pneumococcal Invasive Pneumococcal Disease conjugate vaccine EMD Serono is the biopharmaceutical business of Merck KGaA, Darmstadt Germany in the US Fast Track Designation Orphan Drug Designation Orphan Drug Designation (Greater China) (Greater China) (1)

Achievements and Milestones Clinical data readouts and partnerships provide multiple expected 2022 value drivers for Sutro Achievements and Milestones Clinical data readouts and partnerships provide multiple expected 2022 value drivers for Sutro STRO-002, FolRα ADC Greater China deal with Tasly (Dec. 2021) Ovarian cancer dose-expansion interim data (Jan. 2022) Dose-expansion data with durability (2H 2022) EOP1/2 meeting (Mid-2022) Initiate registration-directed trial in Platinum-Resistant Ovarian Cancer (Early 2023) First patient dosed in endometrial cancer cohort (Nov. 2021) First patient dosed in bevacizumab combination trial (March 2022) Support Tasly for initiation of clinical development activities in Greater China (2022) Initiate clinical trial for NSCLC and other non-gynecologic solid tumors (2H 2022) STRO-001, CD74 ADC Greater China deal with BioNova (Oct. 2021)Support BioNova for initiation of clinical development activities in Greater China (2022)Determine RP2D through dose escalation (2022)Cell-Free Manufacturing for Partnered Programs Provide manufacturing materials & support for CC-99712, BCMA ADC in clinical development (BMS)Manufacture initial product for clinical development of cytokine derivative (Merck)Manufacture M1231 product, MUC1-EGFR ADC in clinical development (EMD Serono)Supply cell-free extract & reagents to Vaxcyte for VAX-24, with first participants dosed in a Phase 2 clinical study Support tech transfers and enable BMS and EMD Serono to manufacture from Sutro’s cell-free extract

FolRα-Targeting ADC Potential Best-in-Class ADC for Ovarian and Endometrial Cancers STRO 002
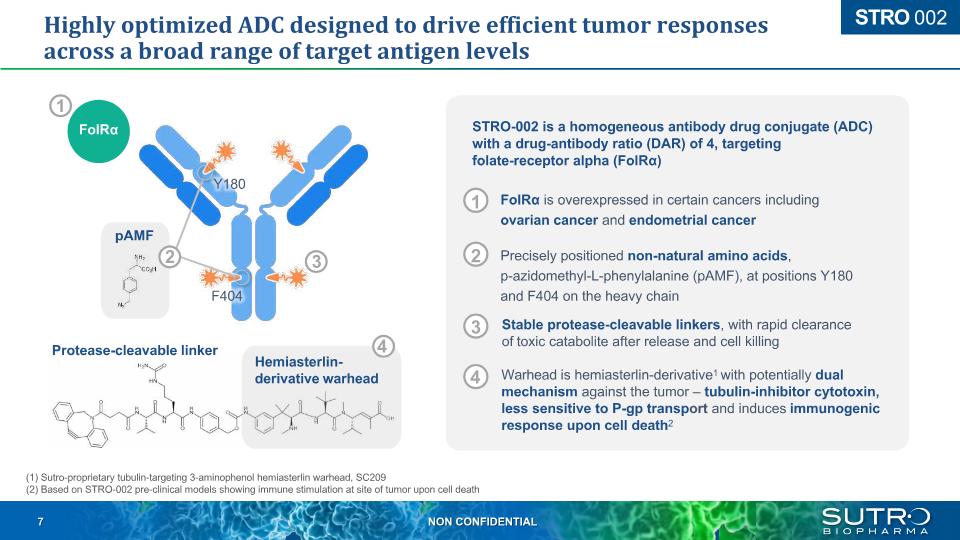
Highly optimized ADC designed to drive efficient tumor responses across a broad range of target antigen levels STRO 002 (1) Sutro-proprietary tubulin-targeting 3-aminophenol hemiasterlin warhead, SC209 (2) Based on STRO-002 pre-clinical models showing immune stimulation at site of tumor upon cell death STRO-002 is a homogeneous antibody drug conjugate (ADC) with a drug-antibody ratio (DAR) of 4, targeting folate-receptor alpha (FolRα) 1 2 3 4 3 Protease-cleavable linker 4 2 pAMF FolRα 1 Hemiasterlin-derivative warhead Y180 F404 FolRα is overexpressed in certain cancers including ovarian cancer and endometrial cancer Precisely positioned non-natural amino acids, p-azidomethyl-L-phenylalanine (pAMF), at positions Y180 and F404 on the heavy chain Stable protease-cleavable linkers, with rapid clearance of toxic catabolite after release and cell killing Warhead is hemiasterlin-derivative1 with potentially dual mechanism against the tumor – tubulin-inhibitor cytotoxin, less sensitive to P-gp transport and induces immunogenic response upon cell death2
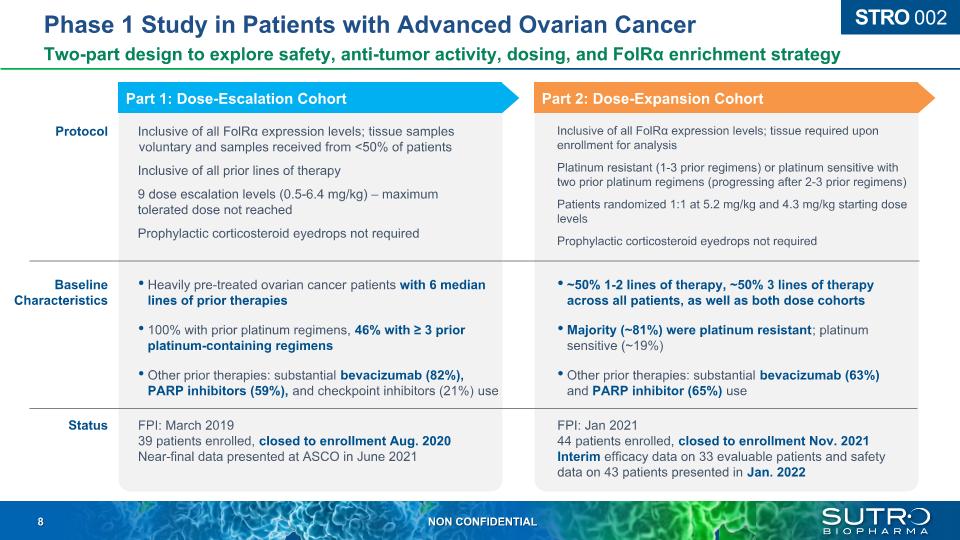
Phase 1 Study in Patients with Advanced Ovarian Cancer Two-part design to explore safety, anti-tumor activity, dosing, and FolRα enrichment strategy STRO 002 Protocol Baseline Characteristics Inclusive of all FolRα expression levels; tissue samples voluntary and samples received from <50% of patients Inclusive of all prior lines of therapy 9 dose escalation levels (0.5-6.4 mg/kg) – maximum tolerated dose not reached Prophylactic corticosteroid eyedrops not required Inclusive of all FolRα expression levels; tissue required upon enrollment for analysis Platinum resistant (1-3 prior regimens) or platinum sensitive with two prior platinum regimens (progressing after 2-3 prior regimens) Patients randomized 1:1 at 5.2 mg/kg and 4.3 mg/kg starting dose levels Prophylactic corticosteroid eyedrops not required ~50% 1-2 lines of therapy, ~50% 3 lines of therapy across all patients, as well as both dose cohorts Majority (~81%) were platinum resistant; platinum sensitive (~19%) Other prior therapies: substantial bevacizumab (63%) and PARP inhibitor (65%) use Heavily pre-treated ovarian cancer patients with 6 median lines of prior therapies 100% with prior platinum regimens, 46% with ≥ 3 prior platinum-containing regimens Other prior therapies: substantial bevacizumab (82%), PARP inhibitors (59%), and checkpoint inhibitors (21%) use Status FPI: March 201939 patients enrolled, closed to enrollment Aug. 2020 Near-final data presented at ASCO in June 2021 FPI: Jan 202144 patients enrolled, closed to enrollment Nov. 2021Interim efficacy data on 33 evaluable patients and safety data on 43 patients presented in Jan. 2022 Part 1: Dose-Escalation Cohort Part 2: Dose-Expansion Cohort
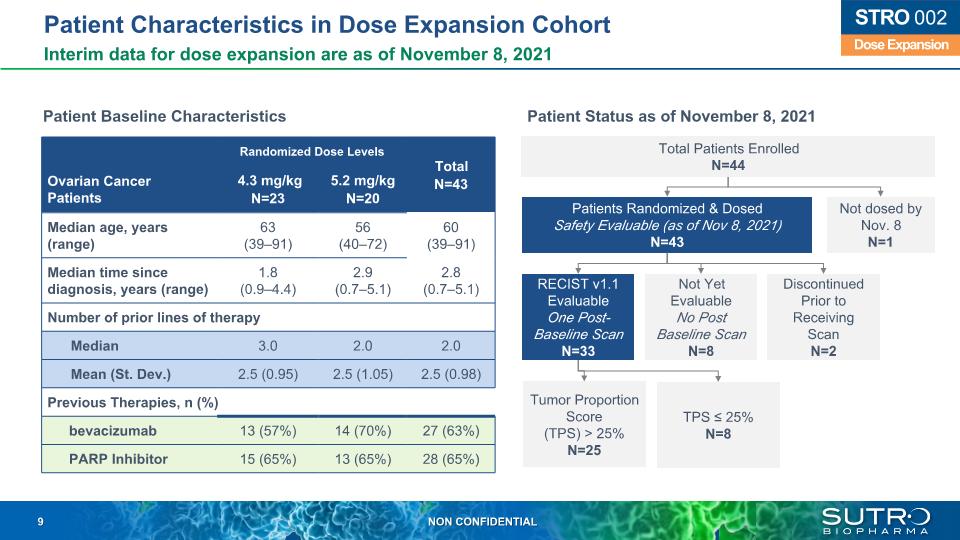
Patient Characteristics in Dose Expansion Cohort Interim data for dose expansion are as of November 8, 2021 Dose Expansion STRO 002 Patients Randomized & Dosed Safety Evaluable (as of Nov 8, 2021) N=43 RECIST v1.1 Evaluable One Post-Baseline Scan N=33 Not Yet Evaluable No Post Baseline Scan N=8 Discontinued Prior to Receiving Scan N=2 Tumor Proportion Score (TPS) > 25% N=25 TPS ≤ 25% N=8 Total Patients Enrolled N=44 Not dosed by Nov. 8 N=1 Randomized Dose Levels Total N=43 Ovarian Cancer Patients 4.3 mg/kg N=23 5.2 mg/kg N=20 Total N=43 Median age, years (range) 63 (39–91) 56 (40–72) 60 (39–91) Median time since diagnosis, years (range) 1.8 (0.9–4.4) 2.9 (0.7–5.1) 2.8 (0.7–5.1) Number of prior lines of therapy Median 3.0 2.0 2.0 Mean (St. Dev.) 2.5 (0.95) 2.5 (1.05) 2.5 (0.98) Previous Therapies, n (%) bevacizumab 13 (57%) 14 (70%) 27 (63%) PARP Inhibitor 15 (65%) 13 (65%) 28 (65%) Patient Status as of November 8, 2021 Patient Baseline Characteristics
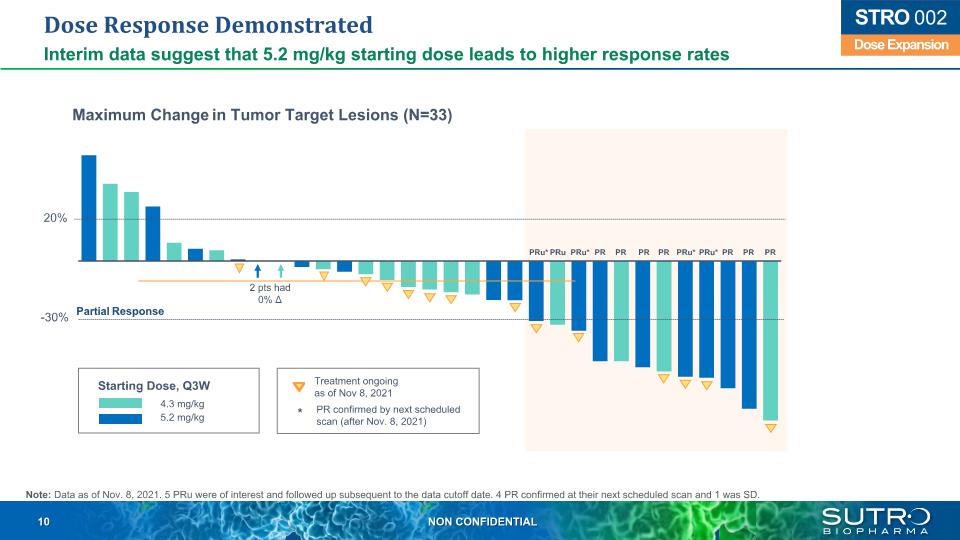
Dose Response Demonstrated Interim data suggest that 5.2 mg/kg starting dose leads to higher response rates 20% -30% 2 pts had 0% Δ Maximum Change in Tumor Target Lesions (N=33) Partial Response 4.3 mg/kg 5.2 mg/kg Treatment ongoingas of Nov 8, 2021 PR confirmed by next scheduled scan (after Nov. 8, 2021) Dose Expansion STRO 002 PRu* PRu PRu* PR PR PR PR PRu* PRu* PR PR PR Starting Dose, Q3W Note: Data as of Nov. 8, 2021. 5 PRu were of interest and followed up subsequent to the data cutoff date. 4 PR confirmed at their next scheduled scan and 1 was SD. *
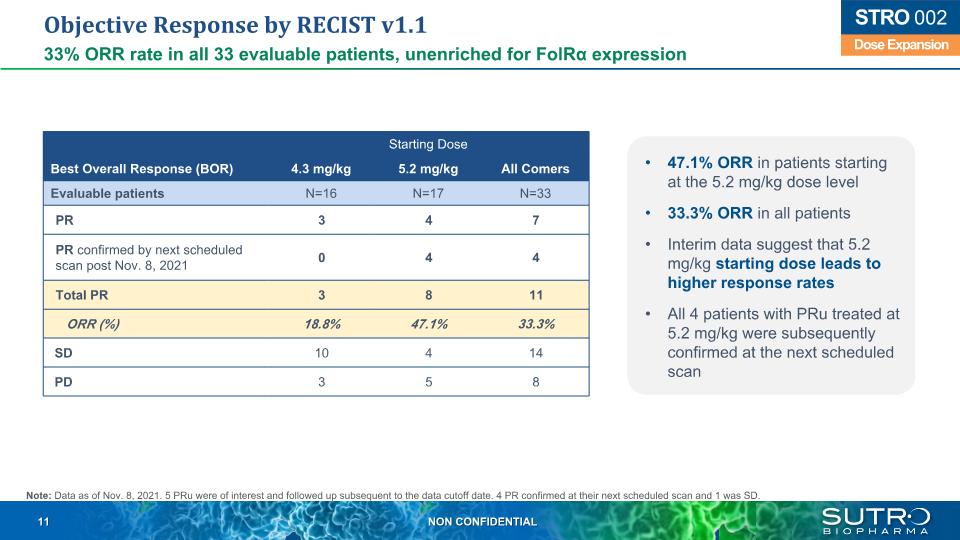
Objective Response by RECIST v1.1 33% ORR rate in all 33 evaluable patients, unenriched for FolRα expression Starting Dose Best Overall Response (BOR) 4.3 mg/kg 5.2 mg/kg All Comers Evaluable patients N=16 N=17 N=33 PR 3 4 7 PR confirmed by next scheduled scan post Nov. 8, 2021 0 4 4 Total PR 3 8 11 ORR (%) 18.8% 47.1% 33.3% SD 10 4 14 PD 3 5 8 Dose Expansion STRO 002 47.1% ORR in patients starting at the 5.2 mg/kg dose level 33.3% ORR in all patients Interim data suggest that 5.2 mg/kg starting dose leads to higher response rates All 4 patients with PRu treated at 5.2 mg/kg were subsequently confirmed at the next scheduled scan Note: Data as of Nov. 8, 2021. 5 PRu were of interest and followed up subsequent to the data cutoff date. 4 PR confirmed at their next scheduled scan and 1 was SD.
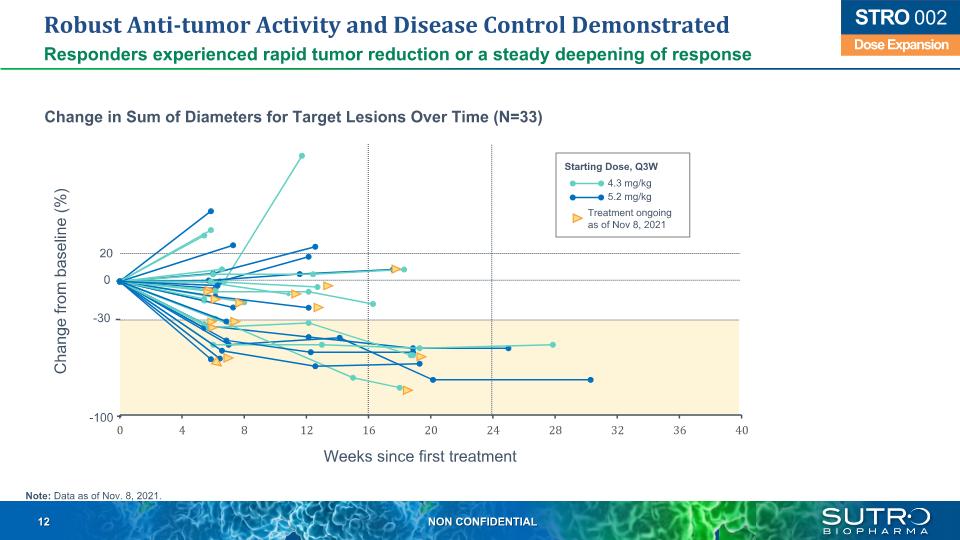
Robust Anti-tumor Activity and Disease Control Demonstrated Responders experienced rapid tumor reduction or a steady deepening of response Dose Expansion STRO 002 Change from baseline (%) Weeks since first treatment 20 -30 -100 0 Change in Sum of Diameters for Target Lesions Over Time (N=33) Starting Dose, Q3W 4.3 mg/kg 5.2 mg/kg Treatment ongoingas of Nov 8, 2021 Note: Data as of Nov. 8, 2021.
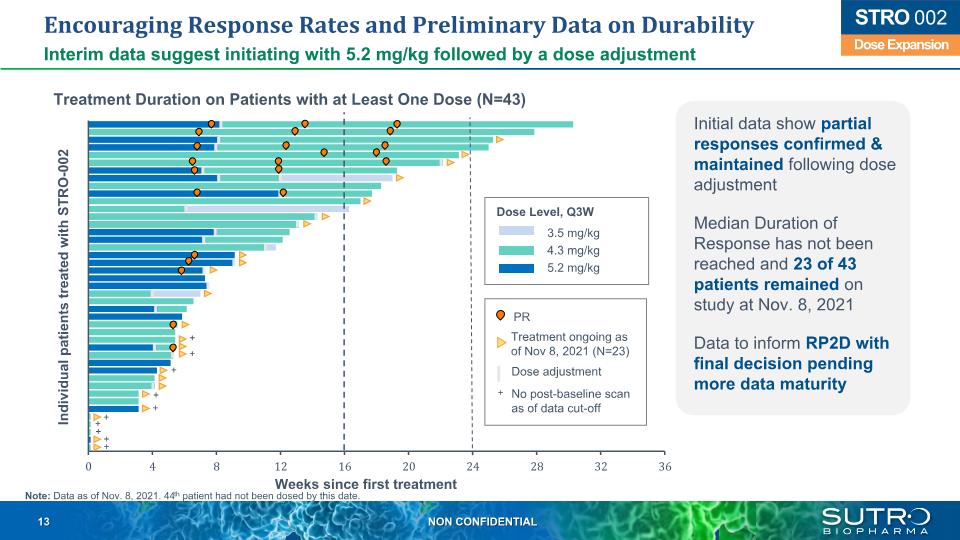
Encouraging Response Rates and Preliminary Data on Durability Interim data suggest initiating with 5.2 mg/kg followed by a dose adjustment Treatment Duration on Patients with at Least One Dose (N=43) Initial data show partial responses confirmed & maintained following dose adjustment Median Duration of Response has not been reached and 23 of 43 patients remained on study at Nov. 8, 2021 Data to inform RP2D with final decision pending more data maturity + Weeks since first treatment Individual patients treated with STRO-002 Dose Level, Q3W 3.5 mg/kg 4.3 mg/kg 5.2 mg/kg + + + + Treatment ongoing as of Nov 8, 2021 (N=23) Dose adjustment PR No post-baseline scan as of data cut-off + + + Note: Data as of Nov. 8, 2021. 44th patient had not been dosed by this date. + + + Dose Expansion STRO 002
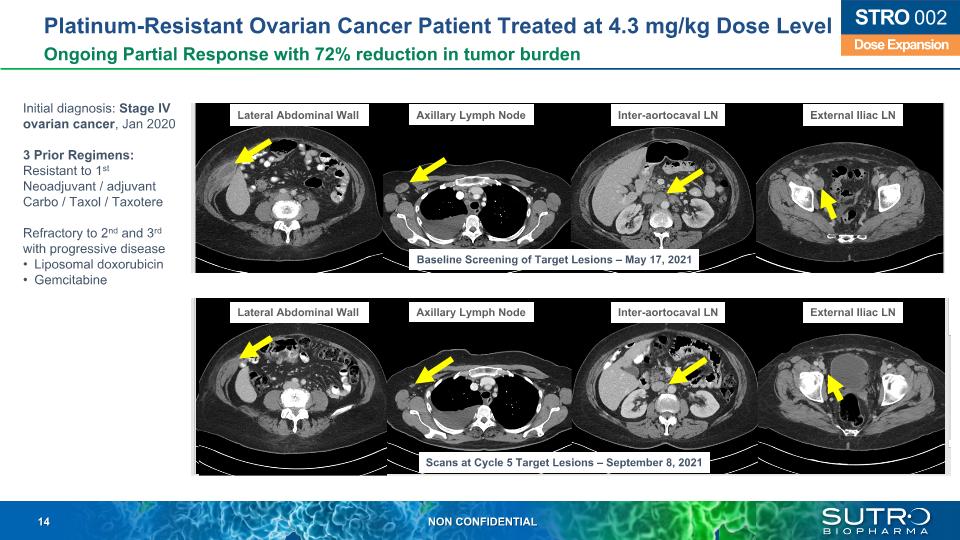
Platinum-Resistant Ovarian Cancer Patient Treated at 4.3 mg/kg Dose Level Ongoing Partial Response with 72% reduction in tumor burden Dose Expansion STRO 002 Initial diagnosis: Stage IV ovarian cancer, Jan 2020 3 Prior Regimens: Resistant to 1st Neoadjuvant / adjuvant Carbo / Taxol / Taxotere Refractory to 2nd and 3rd with progressive disease Liposomal doxorubicin Gemcitabine Lateral Abdominal Wall Axillary Lymph Node Inter-aortocaval LN External Iliac LN Baseline Screening of Target Lesions – May 17, 2021 Scans at Cycle 5 Target Lesions – September 8, 2021 Lateral Abdominal Wall Axillary Lymph Node Inter-aortocaval LN External Iliac LN
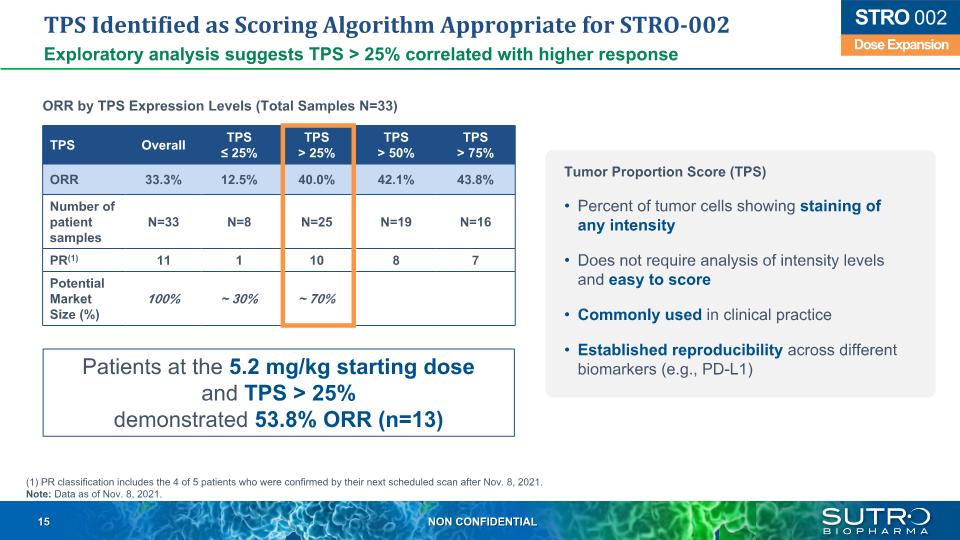
TPS Identified as Scoring Algorithm Appropriate for STRO-002 Exploratory analysis suggests TPS > 25% correlated with higher response Patients at the 5.2 mg/kg starting doseand TPS > 25% demonstrated 53.8% ORR (n=13) ORR by TPS Expression Levels (Total Samples N=33) Dose Expansion STRO 002 TPS Overall TPS ≤ 25% TPS > 25% TPS > 50% TPS> 75% ORR 33.3% 12.5% 40.0% 42.1% 43.8% Number of patient samples N=33 N=8 N=25 N=19 N=16 PR(1) 11 1 10 8 7 Potential Market Size (%) 100% ~ 30% ~ 70% (1) PR classification includes the 4 of 5 patients who were confirmed by their next scheduled scan after Nov. 8, 2021. Note: Data as of Nov. 8, 2021. Tumor Proportion Score (TPS) Percent of tumor cells showing staining of any intensity Does not require analysis of intensity levels and easy to score Commonly used in clinical practice Established reproducibility across different biomarkers (e.g., PD-L1)
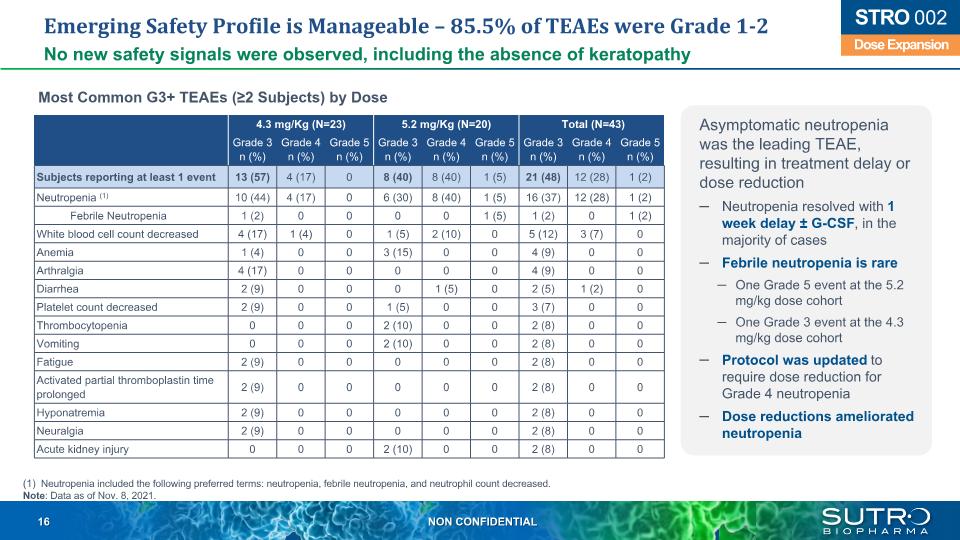
Emerging Safety Profile is Manageable – 85.5% of TEAEs were Grade 1-2 No new safety signals were observed, including the absence of keratopathy 4.3 mg/Kg (N=23) 5.2 mg/Kg (N=20) Total (N=43) Grade 3 n (%) Grade 4 n (%) Grade 5 n (%) Grade 3 n (%) Grade 4 n (%) Grade 5 n (%) Grade 3 n (%) Grade 4 n (%) Grade 5 n (%) Subjects reporting at least 1 event 13 (57) 4 (17) 0 8 (40) 8 (40) 1 (5) 21 (48) 12 (28) 1 (2) Neutropenia (1) 10 (44) 4 (17) 0 6 (30) 8 (40) 1 (5) 16 (37) 12 (28) 1 (2) Febrile Neutropenia 1 (2) 0 0 0 0 1 (5) 1 (2) 0 1 (2) White blood cell count decreased 4 (17) 1 (4) 0 1 (5) 2 (10) 0 5 (12) 3 (7) 0 Anemia 1 (4) 0 0 3 (15) 0 0 4 (9) 0 0 Arthralgia 4 (17) 0 0 0 0 0 4 (9) 0 0 Diarrhea 2 (9) 0 0 0 1 (5) 0 2 (5) 1 (2) 0 Platelet count decreased 2 (9) 0 0 1 (5) 0 0 3 (7) 0 0 Thrombocytopenia 0 0 0 2 (10) 0 0 2 (8) 0 0 Vomiting 0 0 0 2 (10) 0 0 2 (8) 0 0 Fatigue 2 (9) 0 0 0 0 0 2 (8) 0 0 Activated partial thromboplastin time prolonged 2 (9) 0 0 0 0 0 2 (8) 0 0 Hyponatremia 2 (9) 0 0 0 0 0 2 (8) 0 0 Neuralgia 2 (9) 0 0 0 0 0 2 (8) 0 0 Acute kidney injury 0 0 0 2 (10) 0 0 2 (8) 0 0 Neutropenia included the following preferred terms: neutropenia, febrile neutropenia, and neutrophil count decreased. Note: Data as of Nov. 8, 2021. Dose Expansion STRO 002 Asymptomatic neutropenia was the leading TEAE, resulting in treatment delay or dose reduction Neutropenia resolved with 1 week delay ± G-CSF, in the majority of cases Febrile neutropenia is rare One Grade 5 event at the 5.2 mg/kg dose cohort One Grade 3 event at the 4.3 mg/kg dose cohort Protocol was updated to require dose reduction for Grade 4 neutropenia Dose reductions ameliorated neutropenia Most Common G3+ TEAEs (≥2 Subjects) by Dose
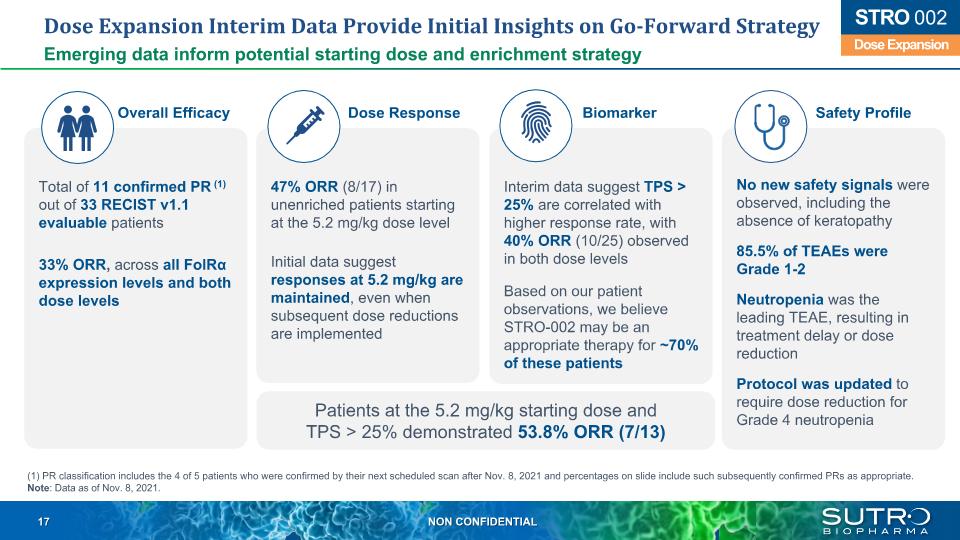
Dose Expansion Interim Data Provide Initial Insights on Go-Forward Strategy Emerging data inform potential starting dose and enrichment strategy Patients at the 5.2 mg/kg starting dose and TPS > 25% demonstrated 53.8% ORR (7/13) Dose Expansion STRO 002 Total of 11 confirmed PR (1) out of 33 RECIST v1.1 evaluable patients 33% ORR, across all FolRα expression levels and both dose levels 47% ORR (8/17) in unenriched patients starting at the 5.2 mg/kg dose level Initial data suggest responses at 5.2 mg/kg are maintained, even when subsequent dose reductions are implemented Interim data suggest TPS > 25% are correlated with higher response rate, with 40% ORR (10/25) observed in both dose levels Based on our patient observations, we believe STRO-002 may be an appropriate therapy for ~70% of these patients No new safety signals were observed, including the absence of keratopathy 85.5% of TEAEs were Grade 1-2 Neutropenia was the leading TEAE, resulting in treatment delay or dose reduction Protocol was updated to require dose reduction for Grade 4 neutropenia Overall Efficacy Dose Response Biomarker Safety Profile (1) PR classification includes the 4 of 5 patients who were confirmed by their next scheduled scan after Nov. 8, 2021 and percentages on slide include such subsequently confirmed PRs as appropriate. Note: Data as of Nov. 8, 2021.
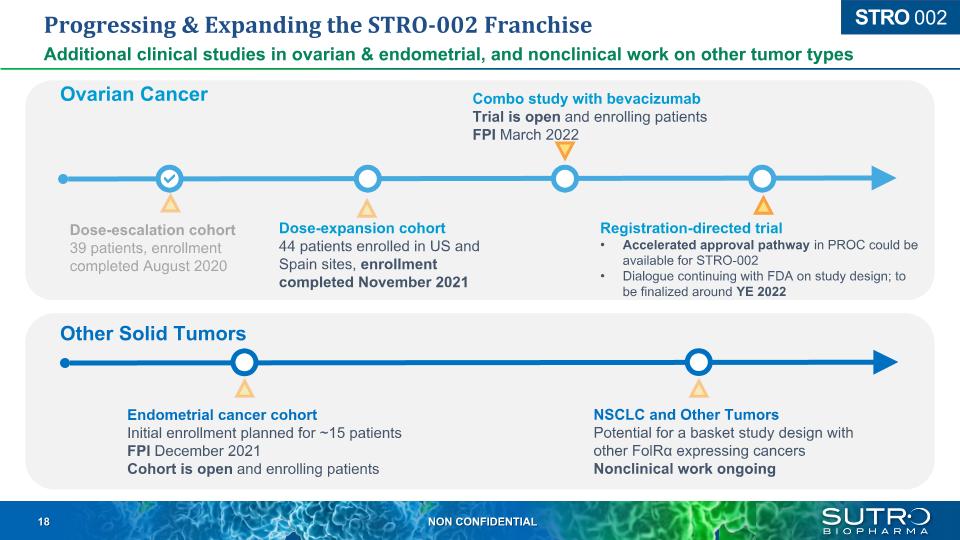
Progressing & Expanding the STRO-002 Franchise Additional clinical studies in ovarian & endometrial, and nonclinical work on other tumor types Dose-escalation cohort 39 patients, enrollment completed August 2020 Dose-expansion cohort44 patients enrolled in US and Spain sites, enrollment completed November 2021 Registration-directed trial Accelerated approval pathway in PROC could be available for STRO-002 Dialogue continuing with FDA on study design; to be finalized around YE 2022 Other Solid Tumors Endometrial cancer cohort Initial enrollment planned for ~15 patients FPI December 2021 Cohort is open and enrolling patients NSCLC and Other Tumors Potential for a basket study design with other FolRα expressing cancers Nonclinical work ongoing STRO 002 Combo study with bevacizumab Trial is open and enrolling patients FPI March 2022 Ovarian Cancer

CD74-Targeting ADC Potential First and Best-in-Class ADC for B-Cell Malignancies STRO 001
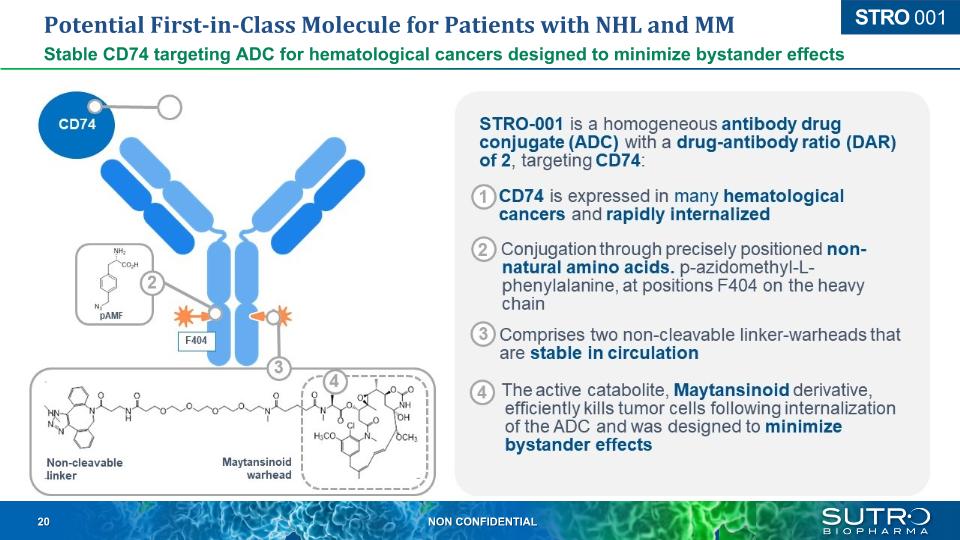
Potential First-in-Class Molecule for Patients with NHL and MM Stable CD74 targeting ADC for hematological cancers designed to minimize bystander effects CD74 STRO 001 CD74 pAMF F404 Non-cleavable linker Maytansinoid warhead STRO-001 is a homogeneous antibody drug conjugate (ADC) with a drug-antibody ratio (DAR) of 2, targeting CD74: CD74 is expressed in many hematological cancers and rapidly internalized Conjugation through precisely positioned non-natural amino acids. p-azidomethyl-L- phenylalanine, at positions F404 on the heavy chain Comprises two non-cleavable linker-warheads that are stable in circulation The active catabolite, Maytansinoid derivative, efficiently kills tumor cells following internalization of the ADC and was designed to minimize bystander effects
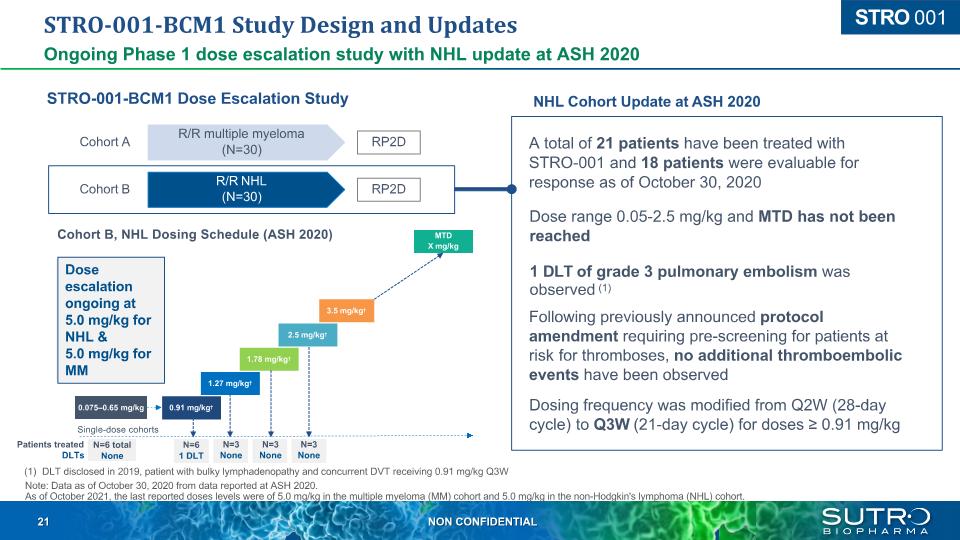
STRO-001-BCM1 Study Design and Updates Ongoing Phase 1 dose escalation study with NHL update at ASH 2020 R/R multiple myeloma (N=30) R/R NHL (N=30) Cohort A Cohort B RP2D RP2D STRO-001-BCM1 Dose Escalation Study NHL Cohort Update at ASH 2020 A total of 21 patients have been treated with STRO-001 and 18 patients were evaluable for response as of October 30, 2020 Dose range 0.05-2.5 mg/kg and MTD has not been reached 1 DLT of grade 3 pulmonary embolism was observed (1) Following previously announced protocol amendment requiring pre-screening for patients at risk for thromboses, no additional thromboembolic events have been observed Dosing frequency was modified from Q2W (28-day cycle) to Q3W (21-day cycle) for doses ≥ 0.91 mg/kg 0.91 mg/kg 1.27 mg/kg 1.78 mg/kg 2.5 mg/kg 3.5 mg/kg MTD X mg/kg 0.075–0.65 mg/kg Single-dose cohorts N=6 total None Patients treated DLTs N=6 1 DLT N=3 None N=3 None N=3 None (1) DLT disclosed in 2019, patient with bulky lymphadenopathy and concurrent DVT receiving 0.91 mg/kg Q3W Note: Data as of October 30, 2020 from data reported at ASH 2020. As of October 2021, the last reported doses levels were of 5.0 mg/kg in the multiple myeloma (MM) cohort and 5.0 mg/kg in the non-Hodgkin's lymphoma (NHL) cohort. Cohort B, NHL Dosing Schedule (ASH 2020) Dose escalation ongoing at 5.0 mg/kg for NHL & 5.0 mg/kg for MM STRO 001
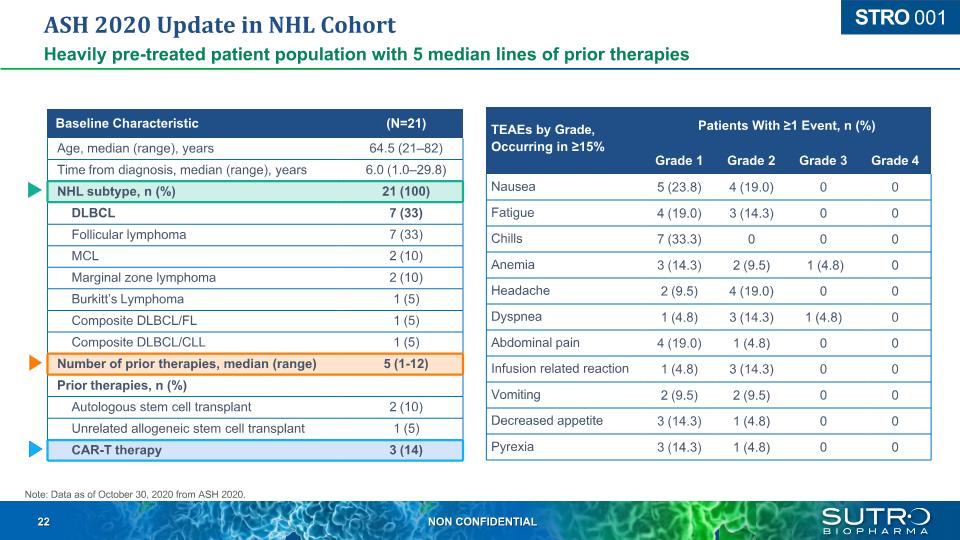
ASH 2020 Update in NHL Cohort Heavily pre-treated patient population with 5 median lines of prior therapies Baseline Characteristic (N=21) Age, median (range), years 64.5 (21–82) Time from diagnosis, median (range), years 6.0 (1.0–29.8) NHL subtype, n (%) 21 (100) DLBCL 7 (33) Follicular lymphoma 7 (33) MCL 2 (10) Marginal zone lymphoma 2 (10) Burkitt’s Lymphoma 1 (5) Composite DLBCL/FL 1 (5) Composite DLBCL/CLL 1 (5) Number of prior therapies, median (range) 5 (1-12) Prior therapies, n (%) Autologous stem cell transplant 2 (10) Unrelated allogeneic stem cell transplant 1 (5) CAR-T therapy 3 (14) TEAEs by Grade, Patients With ≥1 Event, n (%) Occurring in ≥15% Grade 1 Grade 2 Grade 3 Grade 4 Nausea 5 (23.8) 4 (19.0) 0 0 Fatigue 4 (19.0) 3 (14.3) 0 0 Chills 7 (33.3) 0 0 0 Anemia 3 (14.3) 2 (9.5) 1 (4.8) 0 Headache 2 (9.5) 4 (19.0) 0 0 Dyspnea 1 (4.8) 3 (14.3) 1 (4.8) 0 Abdominal pain 4 (19.0) 1 (4.8) 0 0 Infusion related reaction 1 (4.8) 3 (14.3) 0 0 Vomiting 2 (9.5) 2 (9.5) 0 0 Decreased appetite 3 (14.3) 1 (4.8) 0 0 Pyrexia 3 (14.3) 1 (4.8) 0 0 Note: Data as of October 30, 2020 from ASH 2020. STRO 001
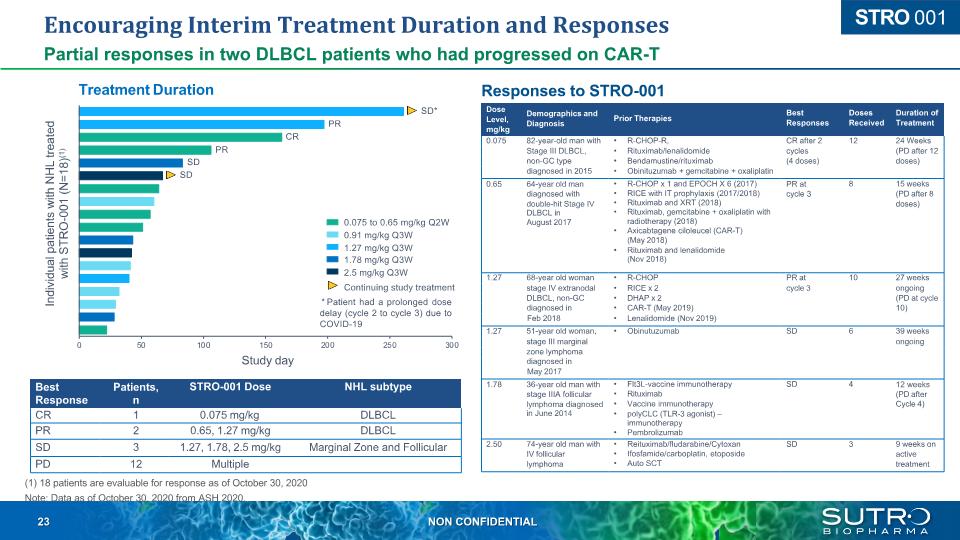
Encouraging Interim Treatment Duration and Responses Partial responses in two DLBCL patients who had progressed on CAR-T Responses to STRO-001 Treatment Duration Best Response Patients, n STRO-001 Dose NHL subtype CR 1 0.075 mg/kg DLBCL PR 2 0.65, 1.27 mg/kg DLBCL SD 3 1.27, 1.78, 2.5 mg/kg Marginal Zone and Follicular PD 12 Multiple (1) 18 patients are evaluable for response as of October 30, 2020 Note: Data as of October 30, 2020 from ASH 2020. Dose Demographics and Level, Diagnosis mg/kg Prior Therapies Best Responses Doses Received Duration of Treatment 0.075 82-year-old man with R-CHOP-R, CR after 2 12 24 Weeks Stage III DLBCL, Rituximab/lenalidomide cycles (PD after 12 non-GC type Bendamustine/rituximab (4 doses) doses) diagnosed in 2015 Obinituzumab + gemcitabine + oxaliplatin 0.65 64-year old man diagnosed with double-hit Stage IV DLBCL in August 2017 R-CHOP x 1 and EPOCH X 6 (2017) RICE with IT prophylaxis (2017/2018) Rituximab and XRT (2018) Rituximab, gemcitabine + oxaliplatin with radiotherapy (2018) Axicabtagene ciloleucel (CAR-T) (May 2018) Rituximab and lenalidomide (Nov 2018) PR at cycle 3 8 15 weeks (PD after 8 doses) 1.27 68-year old woman R-CHOP PR at 10 27 weeks stage IV extranodal RICE x 2 cycle 3 ongoing DLBCL, non-GC DHAP x 2 (PD at cycle diagnosed in CAR-T (May 2019) 10) Feb 2018 Lenalidomide (Nov 2019) 1.27 51-year old woman, Obinutuzumab SD 6 39 weeks stage III marginal ongoing zone lymphoma diagnosed in May 2017 1.78 36-year old man with stage IIIA follicular lymphoma diagnosed in June 2014 Flt3L-vaccine immunotherapy Rituximab Vaccine immunotherapy polyCLC (TLR-3 agonist) – immunotherapy Pembrolizumab SD 4 12 weeks (PD after Cycle 4) 2.50 74-year old man with IV follicular lymphoma Reituximab/fludarabine/Cytoxan Ifosfamide/carboplatin, etoposide Auto SCT SD 3 9 weeks on active treatment 0 50 100 * Patient had a prolonged dose delay (cycle 2 to cycle 3) due to COVID-19 200 250 300 150 Study day Individual patients with NHL treated with STRO-001 (N=18)(1) 0.075 to 0.65 mg/kg Q2W 0.91 mg/kg Q3W 1.27 mg/kg Q3W 1.78 mg/kg Q3W 2.5 mg/kg Q3W Continuing study treatment SD* PR SD SD PR CR STRO 001

Financial Overview Well capitalized through cash and other financial sources $192.1M in cash, cash equivalents & marketable securities as of March 31, 2022 Projected cash runway into 2H 2023(1), based on current business plans and assumptions ~1.6M shares of Vaxcyte (Nasdaq: PCVX) not included in the above reported cash Funding received from our collaborators of ~$456M through March 31, 2022 (1) Based on projections as of March 31, 2022.

Experienced Leadership Team William Newell, JD Chief Executive Officer and Member of the Board of Directors Trevor Hallam, PhD President of Research and Chief Scientific Officer Arturo Molina, MD, MS, FACP Chief Medical Officer Ed Albini, MBA Chief Financial Officer Shabbir Anik, PhD Chief Technical Operations Officer Linda Fitzpatrick Chief People and Communications Officer Nicki Vasquez, PhD Chief Portfolio Strategy and Alliance Officer Jane Chung, RPh Chief Commercial Officer Lynx Neurex