EX-99.1
Published on February 28, 2022
Exhibit 99.1
Sutro Biopharma Reports Full Year 2021 Financial Results, Business Highlights, and Anticipated 2022 Milestones
- Promising STRO-002 initial data on Phase 1 dose-expansion cohort were reported in January 2022, providing initial insights on a go-forward dosing regimen and biomarker enrichment strategy -
- Cash, cash equivalents and marketable securities totaled $229.5 million as of December 31, 2021, with projected cash runway into the second half of 2023 -
SOUTH SAN FRANCISCO, Calif., Feb. 28, 2022 – Sutro Biopharma, Inc. (“Sutro” or the “Company”) (NASDAQ: STRO), a clinical-stage drug discovery, development and manufacturing company focused on the application of precise protein engineering and rational design to create next-generation cancer therapeutics, today reported its financial results for the full year 2021, its recent business highlights, and a preview of anticipated select milestones in 2022.
“We are proud of the achievements at Sutro, as our proprietary cell-free platform has enabled five clinical-stage product candidates, including our internal programs, STRO-002 and STRO-001, and our collaborator programs, CC-99712, M1231, and VAX-24,” said Bill Newell, Sutro’s Chief Executive Officer. “We’ve made significant strides in the development of STRO-002 for ovarian cancer by completing the enrollment of the Phase 1 trial late last year. We intend to work with regulatory agencies on a registrational path forward for patients with ovarian cancer, utilizing our Fast Track Designation. Additionally, we are exploring the therapeutic benefit of STRO-002 in other tumor types. We are currently enrolling patients in an endometrial cohort and plan to initiate a NSCLC study later this year. We continue to explore all strategies to accelerate the development of STRO-002 to provide a potential new therapy for patients suffering from advanced cancers with limited durable treatment options.”
Recent Business Highlights and Anticipated 2022 Select Milestones
STRO-002, FolRα-Targeting Antibody-Drug Conjugate (ADC): STRO-002 is being studied in the clinic, in both the United States and Europe, for patients with ovarian cancer and endometrial cancer.
STRO-001, CD74-Targeting ADC: The Phase 1 study for patients with B‑cell malignancies, including
patients with non-Hodgkin's lymphoma and multiple myeloma, continues in dose escalation.
Additional Pipeline Programs: Research and preclinical development are underway for several internal candidates.
Collaboration Updates: Sutro continues to seek to maximize the value of its proprietary cell-free platform by working with partners on programs in multiple disease spaces and geographies and has received from collaborators an aggregate of approximately $446 million in payments, including equity investments, through December 31, 2021.
Full Year 2021 Financial Highlights
Cash, Cash Equivalents and Marketable Securities
As of December 31, 2021, Sutro had cash, cash equivalents and marketable securities of $229.5 million, as
compared to $326.5 million as of December 31, 2020, with projected runway into the second half of 2023, based on current business plans and assumptions. The above balances do not include the value associated with Sutro’s holdings of Vaxcyte common stock.
Unrealized Loss from Decrease in Value of Vaxcyte Common Stock
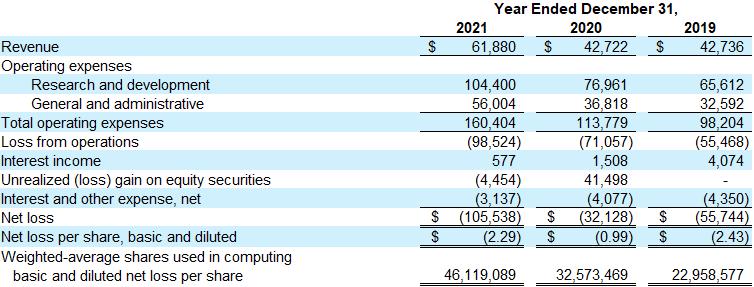
As of December 31, 2021, Sutro held approximately 1.6 million shares of Vaxcyte common stock, with a fair value of $37.2 million. The non-operating, unrealized loss of $4.5 million in 2021 was due to the decrease since December 31, 2020 in the estimated fair value of Sutro’s holdings of Vaxcyte common stock. In 2020, Sutro recorded an unrealized gain of $41.5 million related to its Vaxcyte common stock holdings. Vaxcyte common stock held by Sutro will be remeasured at fair value based on the closing price of Vaxcyte’s common stock on the last trading day of each reporting period, with any non-operating, unrealized gains and losses recorded in Sutro’s statements of operations.
Revenue
Revenue was $61.9 million for the year ended December 31, 2021, as compared to $42.7 million for the same period in 2020, related principally to the Merck, BMS, and EMD Serono collaborations. Future collaboration revenue from Merck, BMS, and EMD Serono, and from any additional collaboration partners, will fluctuate as a result of the amount and timing of revenue recognition of upfront, milestones, and other collaboration agreement payments.
Operating Expenses
Total operating expenses for the year ended December 31, 2021 were $160.4 million, as compared to $113.8 million for the same period in 2020. The 2021 period includes non-cash expenses for stock-based compensation of $23.2 million and depreciation and amortization of $4.8 million, as compared to $11.9 million and $4.3 million, respectively, in the comparable 2020 period. Total operating expenses for the year ended December 31, 2021 were comprised of research and development expenses of $104.4 million and general and administrative expenses of $56.0 million, which are expected to increase in 2022 as Sutro’s internal product candidates advance in clinical development and additional general and administrative expenses are incurred as a public company.
About Sutro Biopharma
Sutro Biopharma, Inc., located in South San Francisco, is a clinical-stage drug discovery, development and manufacturing company. Using precise protein engineering and rational design, Sutro is advancing next-generation oncology therapeutics.
Sutro’s proprietary and integrated cell-free protein synthesis platform XpressCF® and site-specific conjugation platform XpressCF+ led to the discovery of STRO-001 and STRO-002, Sutro’s first two internally-developed ADCs. STRO-001 is a CD74-targeting ADC currently under investigation in a Phase 1 clinical trial for patients with advanced B-cell malignancies and was granted Orphan Drug Designation by the FDA for multiple myeloma. STRO-002, a folate receptor alpha (FolRα)-targeting ADC, is currently being investigated in a Phase 1 clinical trial for patients with ovarian and endometrial cancers and was granted Fast Track designation by the FDA for ovarian cancer. A third product candidate, CC-99712, a BCMA-targeting ADC, which is part of Sutro’s collaboration with Bristol Myers Squibb, formerly Celgene Corporation, is enrolling patients for its Phase 1 clinical trial of patients with multiple myeloma and has
received Orphan Drug Designation from the FDA. A fourth product candidate, M1231, a MUC1-EGFR, bispecific ADC, which is part of Sutro’s collaboration with Merck KGaA, Darmstadt, Germany, known as EMD Serono in the U.S. and Canada (EMD Serono), is enrolling patients for its Phase 1 clinical trial of patients with metastatic solid tumors, non-small cell lung cancer (NSCLC) and esophageal squamous cell carcinoma. These four product candidates resulted from Sutro’s XpressCF® and XpressCF+ technology platforms. Bristol Myers Squibb and EMD Serono have worldwide development and commercialization rights for CC-99712 and M1231, respectively, for which Sutro is entitled to milestone or contingent payments and tiered royalties.
Sutro is dedicated to transforming the lives of cancer patients by creating medicines with improved therapeutic profiles for areas of unmet need. To date, Sutro’s platform has led to ADCs, bispecific antibodies, cytokine-based immuno-oncology therapies, and vaccines directed at precedented targets in clinical indications where the current standard of care is suboptimal.
Sutro’s platform allows it to accelerate discovery and development of potential first-in-class and best-in-class molecules through rapid and systematic evaluation of protein structure-activity relationships to create optimized homogeneous product candidates. In addition to developing its own oncology pipeline, Sutro is collaborating with select pharmaceutical and biotechnology companies to discover and develop novel, next-generation therapeutics.
Follow Sutro on Twitter, @Sutrobio, and at www.sutrobio.com to learn more about our passion for changing the future of oncology.
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the “safe harbor” provisions of the Private Securities Litigation Reform Act of 1995, including, but not limited to, anticipated preclinical and clinical development activities, timing of announcements of clinical results, potential benefits of STRO-002 and the Company’s other product candidates and platform, potential future milestone and royalty payments, and potential market opportunities for STRO-002 and the Company’s other product candidates. All statements other than statements of historical fact are statements that could be deemed forward-looking statements. Although the Company believes that the expectations reflected in such forward-looking statements are reasonable, the Company cannot guarantee future events, results, actions, levels of activity, performance or achievements, and the timing and results of biotechnology development and potential regulatory approval is inherently uncertain. Forward-looking statements are subject to risks and uncertainties that may cause the Company’s actual activities or results to differ significantly from those expressed in any forward-looking statement, including risks and uncertainties related to the Company’s ability to advance its product candidates, the receipt and timing of potential regulatory designations, approvals and commercialization of product candidates and the Company’s ability to successfully leverage Fast Track designation, the market size for the Company’s product candidates to be smaller than anticipated, the impact of the COVID-19 pandemic on the Company’s business, clinical trial sites, supply chain and manufacturing facilities, the Company’s ability to maintain and recognize the benefits of certain designations received by product candidates, the timing and results of preclinical and clinical trials, the Company’s ability to fund development activities and achieve development goals, the Company’s ability to protect intellectual property, the value of the Company’s holdings of Vaxcyte common stock, and the
Company’s commercial collaborations with third parties and other risks and uncertainties described under the heading “Risk Factors” in documents the Company files from time to time with the Securities and Exchange Commission. These forward-looking statements speak only as of the date of this press release, and the Company undertakes no obligation to revise or update any forward-looking statements to reflect events or circumstances after the date hereof.
Investor Contact
Annie J. Chang
Sutro Biopharma
(650) 801-5728
ajchang@sutrobio.com
Media Contact
Maggie Beller
Russo Partners
(646) 942-5631
Maggie.beller@russopartnersllc.com
Sutro Biopharma, Inc.
Selected Statements of Operations Financial Data
(Unaudited)
(In thousands, except per share amounts)
Sutro Biopharma, Inc.
Selected Balance Sheet Financial Data
(Unaudited)
(In thousands) 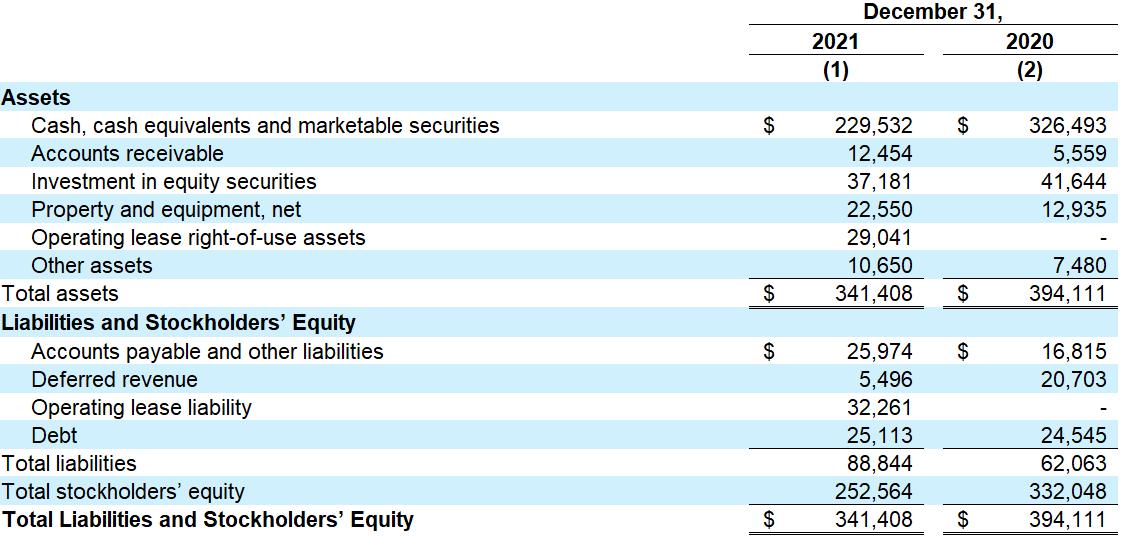
(1) The condensed balance sheet as of December 31, 2021 was derived from the audited financial statements included in the Company's Annual Report on Form 10-K for the year ended December 31, 2021, filed with the Securities and Exchange Commission on February 28, 2022.
(2) The condensed balance sheet as of December 31, 2020 was derived from the audited financial statements included in the Company's Annual Report on Form 10-K for the year ended December 31, 2020, filed with the Securities and Exchange Commission on March 18, 2021.