EX-99.2
Published on January 5, 2022

NON-CONFIDENTIAL 2022 KOL Discussion of STRO-002 Phase 1 Interim Dose Expansion Data January 5, 20225:00pm ET / 2:00pm PT Sutro Biopharma “NASDAQ: STRO Exhibit 99.2

Forward Looking Statements This presentation and the accompanying oral presentation contain “forward-looking” statements that are based on our management’s beliefs and assumptions and on information currently available to management. Forward-looking statements include all statements other than statements of historical fact contained in this presentation, including information concerning our future financial performance, business plans and objectives, current and future clinical activities, timing and success of our ongoing and planned clinical trials and related data, updates and results of our clinical trials and related data, timing and success of our planned development activities, our ability to obtain and maintain regulatory approval, the potential therapeutic benefits and economic value of our product candidates, potential growth opportunities, financing plans, competitive position, industry environment and potential market opportunities. Forward-looking statements are subject to known and unknown risks, uncertainties, assumptions and other factors, including risks and uncertainties related to our cash forecasts, our and our collaborators’ ability to advance our product candidates, the receipt and timing of potential regulatory submissions, designations, approvals and commercialization of product candidates, the timing and results of preclinical and clinical trials, and the expected impact of the COVID-19 pandemic on our operations. It is not possible for our management to predict all risks, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements we may make. These factors, together with those that may be described in greater detail under the heading “Risk Factors” contained in our most recent Annual Report on Form 10-K, Quarterly Report on Form 10-Q and other reports the company files from time to time with the Securities and Exchange Commission, may cause our actual results, performance or achievements to differ materially and adversely from those anticipated or implied by our forward-looking statements. You should not rely upon forward-looking statements as predictions of future events. Although our management believes that the expectations reflected in our forward- looking statements are reasonable, we cannot guarantee that the future results, levels of activity, performance or events and circumstances described in the forward- looking statements will be achieved or occur. Moreover, neither we nor our management assume responsibility for the accuracy and completeness of the forward- looking statements. We undertake no obligation to publicly update any forward-looking statements for any reason after the date of this presentation to conform these statements to actual results or to changes in our expectations, except as required by law. This presentation also contains estimates and other statistical data made by independent parties and by us relating to market size and growth and other data about our industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. In addition, projections, assumptions, and estimates of our future performance and the future performance of the markets in which we operate are necessarily subject to a high degree of uncertainty and risk.

Agenda for Today Topic Speakers Opening Comments Welcome & Agenda Review Forward-Looking Statements Ed Albini, Chief Financial Officer CEO Opening Comments Study Objectives and Overview Bill Newell, Chief Executive Officer Data Presentation Study Objectives and Overview STRO-002 Phase 1 Dose Expansion Interim Data R. Wendel Naumann, M.D., Professor & Director of Gynecologic Oncology Research, Associate Medical Director of Clinical Trials, at Levine Cancer Institute, Atrium Health Arturo Molina, M.D., MS, FACP, Chief Medical Officer Summary Next Steps for STRO-002 Arturo Molina, M.D., MS, FACP Closing Bill Newell Q&A R. Wendel Naumann, M.D. Bill Newell Arturo Molina, M.D., MS, FACP Trevor Hallam, Ph.D., President of Research and Chief Scientific Officer Ed Albini Today’s Agenda January 5, 2022
Agenda for Today Welcome Dr. R. Wendel Naumann Phase 1 STRO-002-GM1 Co-Principal Investigator Dr. Naumann is currently the Director of Minimally Invasive Surgery in Gynecologic Oncology and Professor in the Department of Ob/Gyn at the Levine Cancer Institute, Atrium Health. Dr. Naumann did his residency in Obstetrics and Gynecology as well as his fellowship in Gynecologic Oncology at the University of Alabama School of Medicine in Birmingham. Dr. Naumann has served as a board member on the Executive Council of the Society of Gynecologic Oncology (SGO) and the Chair of Education Committee and is currently the co-director of the SGO Winter meeting. He has an interest in chemotherapy development including targeted therapies and immune therapies and runs the phase I trials in gynecologic oncology at the Levine Cancer Institute. He has served as a member of the GOG/NRG corpus committee and the Developmental Therapeutics committee. R. Wendel Naumann, M.D. Professor & Director of Gynecologic Oncology Research Associate Medical Director of Clinical Trials,Levine Cancer Institute, Atrium Health Sutro Biopharma Clinical Advisory Board
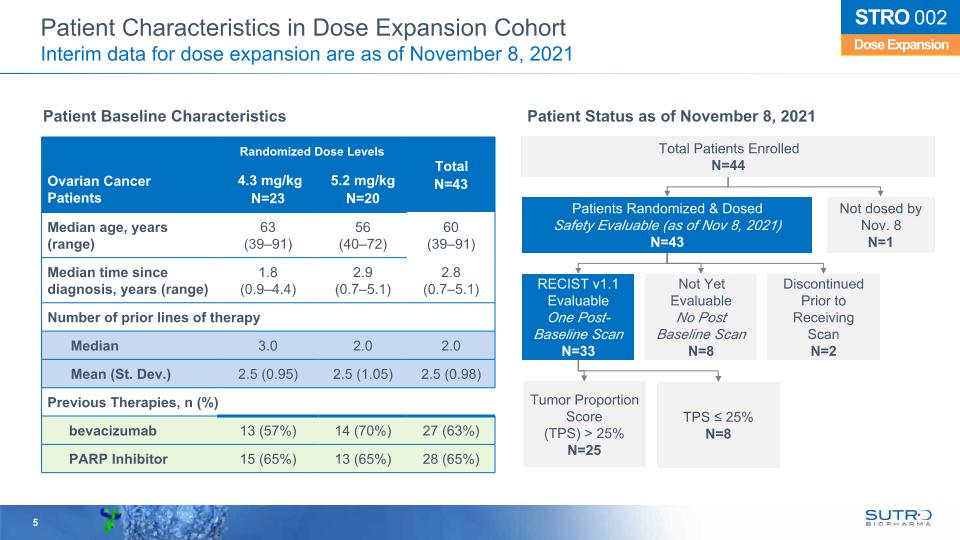
5 Dose Expansion STRO 002 Patient Characteristics in Dose Expansion Cohort Interim data for dose expansion are as of November 8, 2021 Patients Randomized & Dosed Safety Evaluable (as of Nov 8, 2021) N=43 RECIST v1.1 Evaluable One Post-Baseline Scan N=33 Not Yet Evaluable No Post Baseline Scan N=8 Discontinued Prior to Receiving Scan N=2 Tumor Proportion Score (TPS) > 25% N=25 TPS ≤ 25% N=8 Total Patients Enrolled N=44 Not dosed by Nov. 8 N=1 Randomized Dose Levels Total N=43 Ovarian Cancer Patients 4.3 mg/kg N=23 5.2 mg/kg N=20 Total N=43 Median age, years (range) 63 (39–91) 56 (40–72) 60 (39–91) Median time since diagnosis, years (range) 1.8 (0.9–4.4) 2.9 (0.7–5.1) 2.8 (0.7–5.1) Number of prior lines of therapy Median 3.0 2.0 2.0 Mean (St. Dev.) 2.5 (0.95) 2.5 (1.05) 2.5 (0.98) Previous Therapies, n (%) bevacizumab 13 (57%) 14 (70%) 27 (63%) PARP Inhibitor 15 (65%) 13 (65%) 28 (65%) Patient Status as of November 8, 2021 Patient Baseline Characteristics
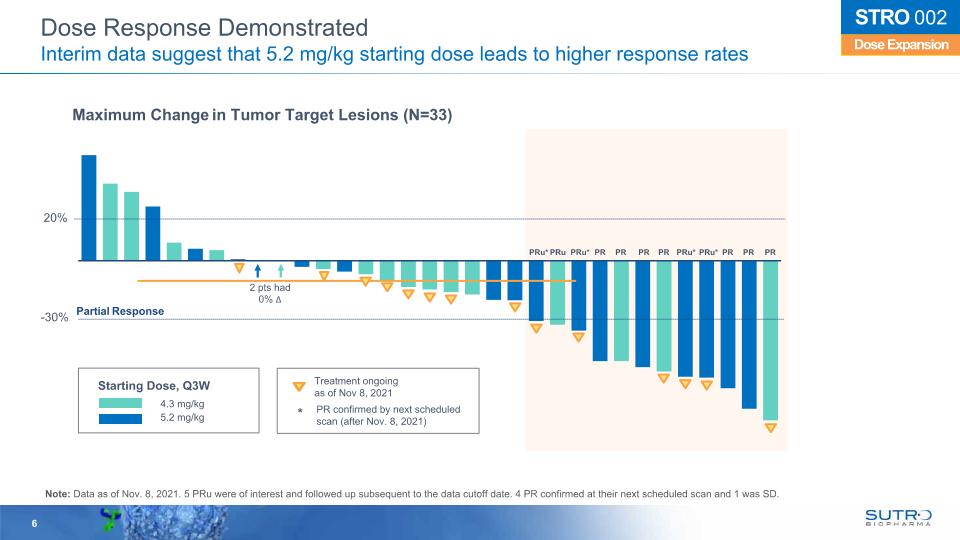
6 20% -30% 2 pts had 0% Δ Maximum Change in Tumor Target Lesions (N=33) Partial Response 4.3 mg/kg 5.2 mg/kg Treatment ongoingas of Nov 8, 2021 PR confirmed by next scheduled scan (after Nov. 8, 2021) Dose Expansion STRO 002 Dose Response Demonstrated Interim data suggest that 5.2 mg/kg starting dose leads to higher response rates PRu* PRu PRu* PR PR PR PR PRu* PRu* PR PR PR Starting Dose, Q3W Note: Data as of Nov. 8, 2021. 5 PRu were of interest and followed up subsequent to the data cutoff date. 4 PR confirmed at their next scheduled scan and 1 was SD. *
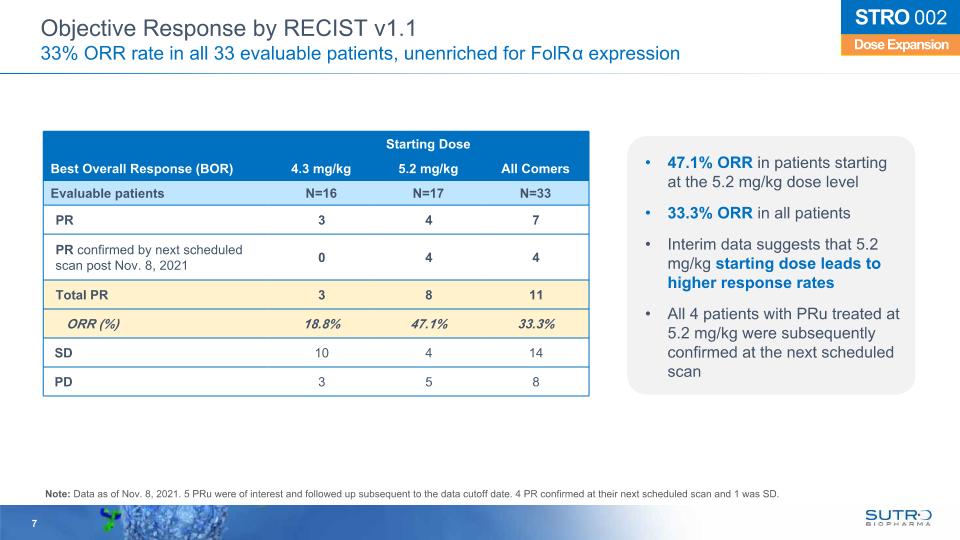
Starting Dose Best Overall Response (BOR) 4.3 mg/kg 5.2 mg/kg All Comers Evaluable patients N=16 N=17 N=33 PR 3 4 7 PR confirmed by next scheduled scan post Nov. 8, 2021 0 4 4 Total PR 3 8 11 ORR (%) 18.8% 47.1% 33.3% SD 10 4 14 PD 3 5 8 7 Dose Expansion STRO 002 Objective Response by RECIST v1.1 33% ORR rate in all 33 evaluable patients, unenriched for FolRα expression 47.1% ORR in patients starting at the 5.2 mg/kg dose level 33.3% ORR in all patients Interim data suggests that 5.2 mg/kg starting dose leads to higher response rates All 4 patients with PRu treated at 5.2 mg/kg were subsequently confirmed at the next scheduled scan Note: Data as of Nov. 8, 2021. 5 PRu were of interest and followed up subsequent to the data cutoff date. 4 PR confirmed at their next scheduled scan and 1 was SD.
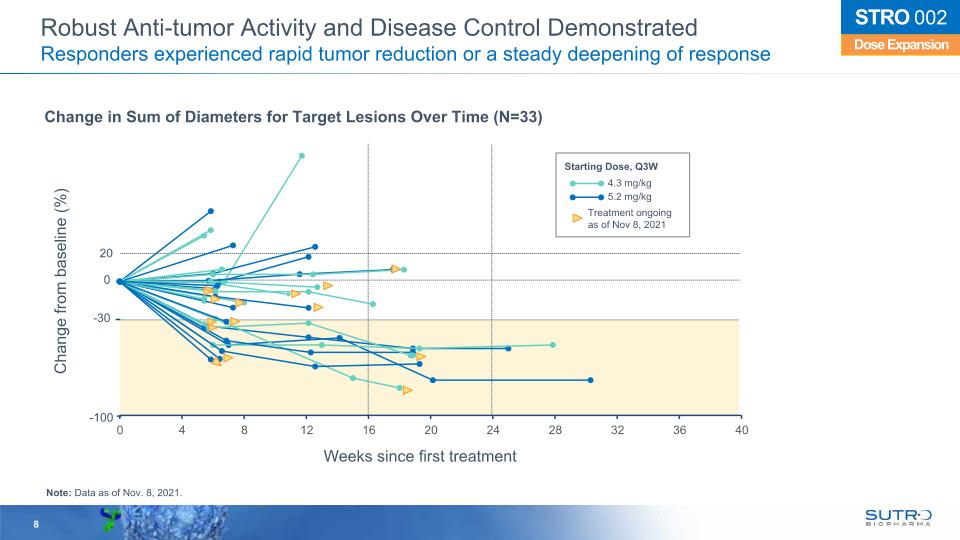
Dose Expansion STRO 002 Robust Anti-tumor Activity and Disease Control Demonstrated Responders experienced rapid tumor reduction or a steady deepening of response Change from baseline (%) Weeks since first treatment 20 -30 -100 0 Change in Sum of Diameters for Target Lesions Over Time (N=33) Starting Dose, Q3W 4.3 mg/kg 5.2 mg/kg Treatment ongoingas of Nov 8, 2021 Note: Data as of Nov. 8, 2021.
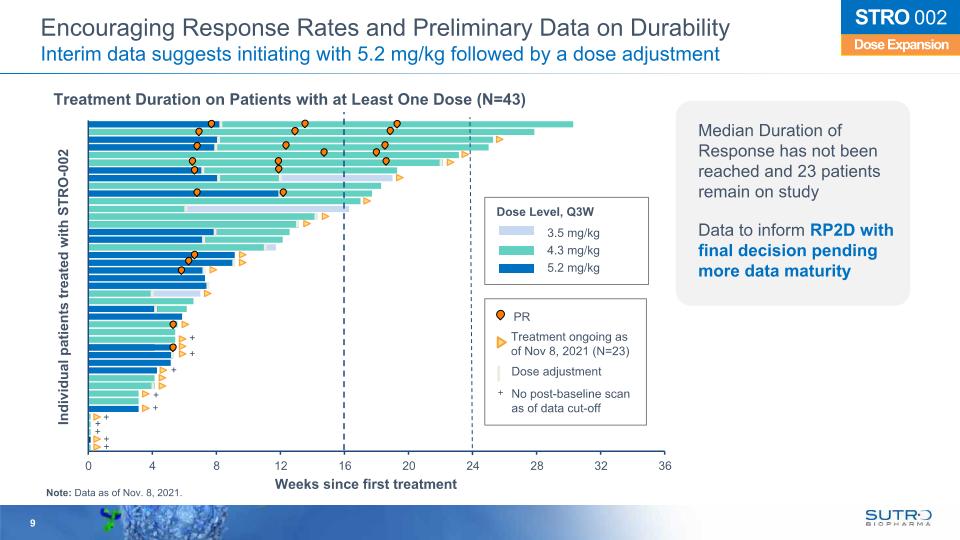
Encouraging Response Rates and Preliminary Data on Durability Interim data suggests initiating with 5.2 mg/kg followed by a dose adjustment Treatment Duration on Patients with at Least One Dose (N=43) Dose Expansion STRO 002 Median Duration of Response has not been reached and 23 patients remain on study Data to inform RP2D with final decision pending more data maturity + Weeks since first treatment Individual patients treated with STRO-002 Dose Level, Q3W 3.5 mg/kg 4.3 mg/kg 5.2 mg/kg + + + + Treatment ongoing as of Nov 8, 2021 (N=23) Dose adjustment PR No post-baseline scan as of data cut-off + + + Note: Data as of Nov. 8, 2021. + + +
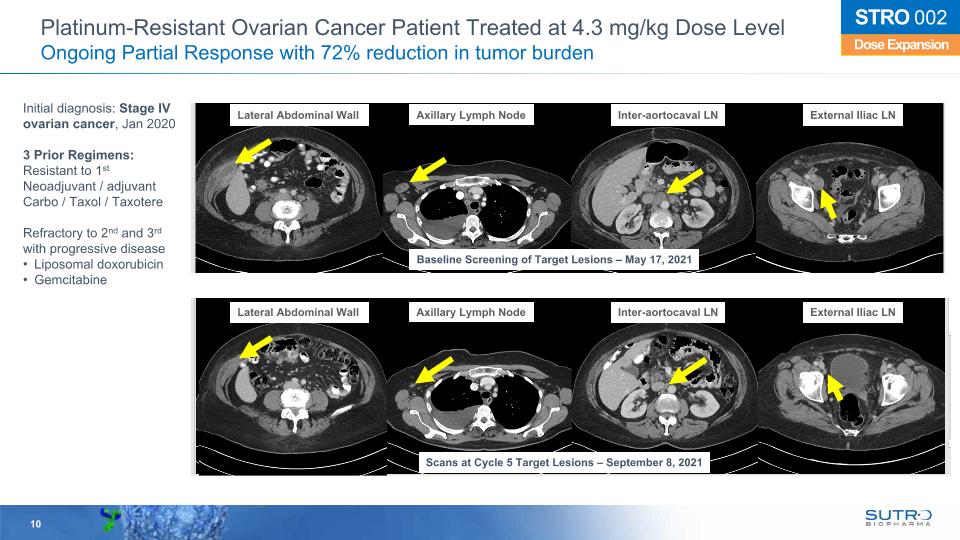
Platinum-Resistant Ovarian Cancer Patient Treated at 4.3 mg/kg Dose Level Ongoing Partial Response with 72% reduction in tumor burden Dose Expansion STRO 002 Initial diagnosis: Stage IV ovarian cancer, Jan 2020 3 Prior Regimens: Resistant to 1st Neoadjuvant / adjuvant Carbo / Taxol / Taxotere Refractory to 2nd and 3rd with progressive disease Liposomal doxorubicin Gemcitabine Lateral Abdominal Wall Axillary Lymph Node Inter-aortocaval LN External Iliac LN Baseline Screening of Target Lesions – May 17, 2021 Scans at Cycle 5 Target Lesions – September 8, 2021 Lateral Abdominal Wall Axillary Lymph Node Inter-aortocaval LN External Iliac LN
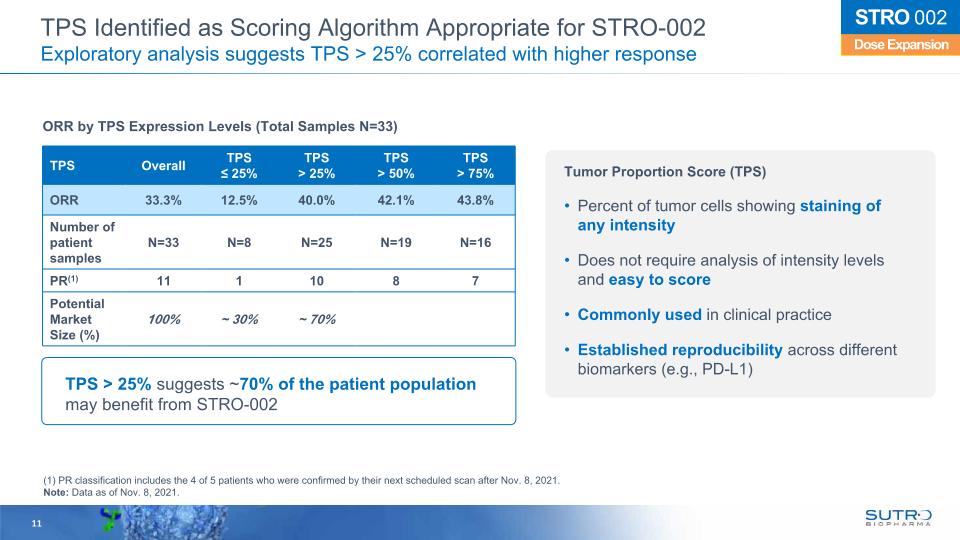
11 TPS > 25% suggests ~70% of the patient population may benefit from STRO-002 ORR by TPS Expression Levels (Total Samples N=33) Dose Expansion STRO 002 TPS Overall TPS ≤ 25% TPS > 25% TPS > 50% TPS> 75% ORR 33.3% 12.5% 40.0% 42.1% 43.8% Number of patient samples N=33 N=8 N=25 N=19 N=16 PR(1) 11 1 10 8 7 Potential Market Size (%) 100% ~ 30% ~ 70% (1) PR classification includes the 4 of 5 patients who were confirmed by their next scheduled scan after Nov. 8, 2021. Note: Data as of Nov. 8, 2021. TPS Identified as Scoring Algorithm Appropriate for STRO-002 Exploratory analysis suggests TPS > 25% correlated with higher response Tumor Proportion Score (TPS) Percent of tumor cells showing staining of any intensity Does not require analysis of intensity levels and easy to score Commonly used in clinical practice Established reproducibility across different biomarkers (e.g., PD-L1)
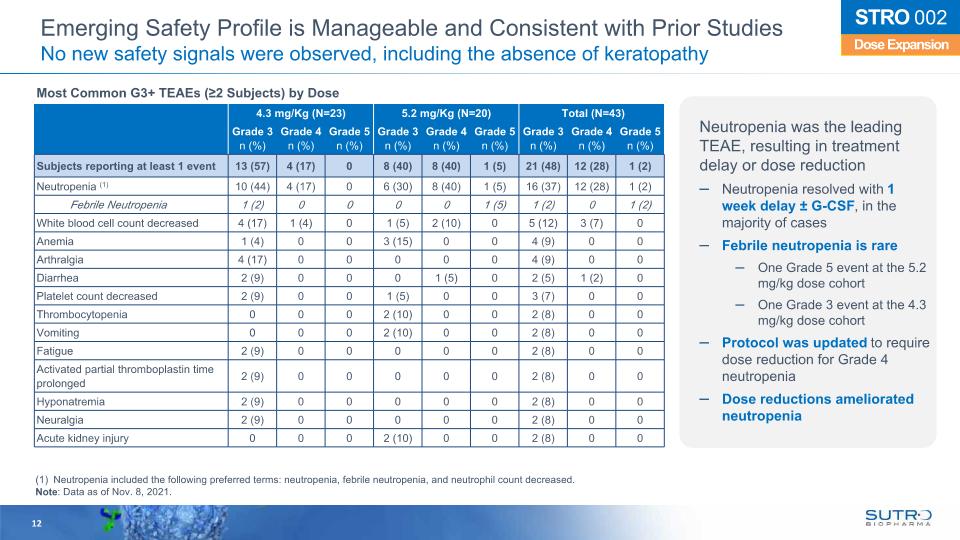
Emerging Safety Profile is Manageable and Consistent with Prior Studies No new safety signals were observed, including the absence of keratopathy 4.3 mg/Kg (N=23) 5.2 mg/Kg (N=20) Total (N=43) Grade 3 n (%) Grade 4 n (%) Grade 5 n (%) Grade 3 n (%) Grade 4 n (%) Grade 5 n (%) Grade 3 n (%) Grade 4 n (%) Grade 5 n (%) Subjects reporting at least 1 event 13 (57) 4 (17) 0 8 (40) 8 (40) 1 (5) 21 (48) 12 (28) 1 (2) Neutropenia (1) 10 (44) 4 (17) 0 6 (30) 8 (40) 1 (5) 16 (37) 12 (28) 1 (2) Febrile Neutropenia 1 (2) 0 0 0 0 1 (5) 1 (2) 0 1 (2) White blood cell count decreased 4 (17) 1 (4) 0 1 (5) 2 (10) 0 5 (12) 3 (7) 0 Anemia 1 (4) 0 0 3 (15) 0 0 4 (9) 0 0 Arthralgia 4 (17) 0 0 0 0 0 4 (9) 0 0 Diarrhea 2 (9) 0 0 0 1 (5) 0 2 (5) 1 (2) 0 Platelet count decreased 2 (9) 0 0 1 (5) 0 0 3 (7) 0 0 Thrombocytopenia 0 0 0 2 (10) 0 0 2 (8) 0 0 Vomiting 0 0 0 2 (10) 0 0 2 (8) 0 0 Fatigue 2 (9) 0 0 0 0 0 2 (8) 0 0 Activated partial thromboplastin time prolonged 2 (9) 0 0 0 0 0 2 (8) 0 0 Hyponatremia 2 (9) 0 0 0 0 0 2 (8) 0 0 Neuralgia 2 (9) 0 0 0 0 0 2 (8) 0 0 Acute kidney injury 0 0 0 2 (10) 0 0 2 (8) 0 0 Neutropenia included the following preferred terms: neutropenia, febrile neutropenia, and neutrophil count decreased. Note: Data as of Nov. 8, 2021. 12 Dose Expansion STRO 002 Most Common G3+ TEAEs (≥2 Subjects) by Dose Neutropenia was the leading TEAE, resulting in treatment delay or dose reduction Neutropenia resolved with 1 week delay ± G-CSF, in the majority of cases Febrile neutropenia is rare One Grade 5 event at the 5.2 mg/kg dose cohort One Grade 3 event at the 4.3 mg/kg dose cohort Protocol was updated to require dose reduction for Grade 4 neutropenia Dose reductions ameliorated neutropenia
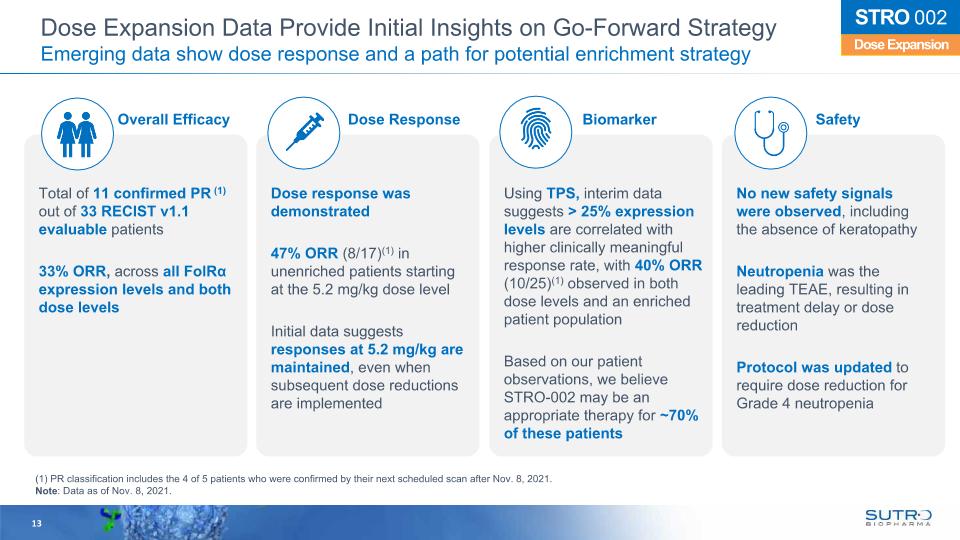
13 Dose Expansion STRO 002 Dose Expansion Data Provide Initial Insights on Go-Forward Strategy Emerging data show dose response and a path for potential enrichment strategy Total of 11 confirmed PR (1) out of 33 RECIST v1.1 evaluable patients 33% ORR, across all FolRα expression levels and both dose levels Dose response was demonstrated 47% ORR (8/17)(1) in unenriched patients starting at the 5.2 mg/kg dose level Initial data suggests responses at 5.2 mg/kg are maintained, even when subsequent dose reductions are implemented Using TPS, interim data suggests > 25% expression levels are correlated with higher clinically meaningful response rate, with 40% ORR (10/25)(1) observed in both dose levels and an enriched patient population Based on our patient observations, we believe STRO-002 may be an appropriate therapy for ~70% of these patients No new safety signals were observed, including the absence of keratopathy Neutropenia was the leading TEAE, resulting in treatment delay or dose reduction Protocol was updated to require dose reduction for Grade 4 neutropenia Overall Efficacy Dose Response Biomarker Safety (1) PR classification includes the 4 of 5 patients who were confirmed by their next scheduled scan after Nov. 8, 2021. Note: Data as of Nov. 8, 2021.
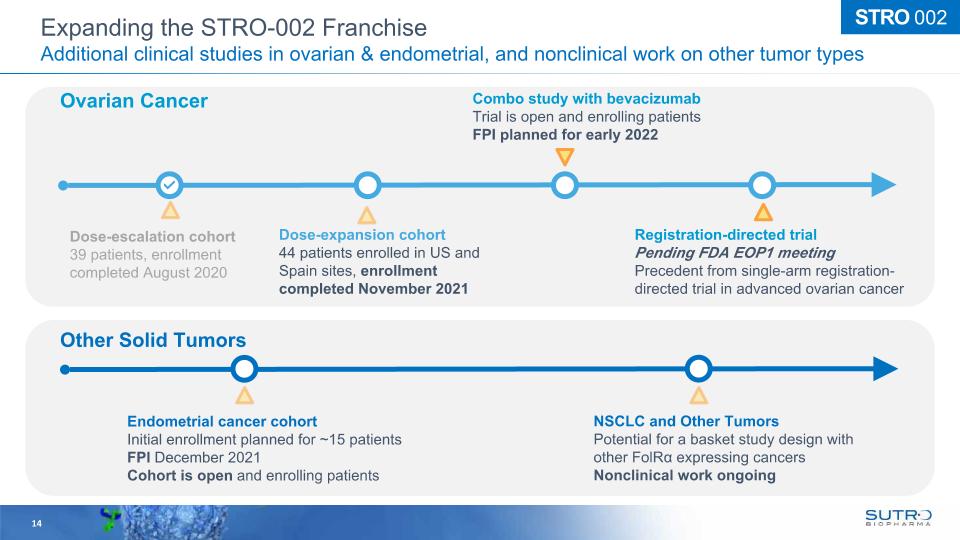
Dose-escalation cohort 39 patients, enrollment completed August 2020 Dose-expansion cohort44 patients enrolled in US and Spain sites, enrollment completed November 2021 Combo study with bevacizumab Trial is open and enrolling patients FPI planned for early 2022 Registration-directed trial Pending FDA EOP1 meeting Precedent from single-arm registration-directed trial in advanced ovarian cancer Ovarian Cancer Other Solid Tumors Endometrial cancer cohort Initial enrollment planned for ~15 patients FPI December 2021 Cohort is open and enrolling patients NSCLC and Other Tumors Potential for a basket study design with other FolRα expressing cancers Nonclinical work ongoing 14 STRO 002 Expanding the STRO-002 Franchise Additional clinical studies in ovarian & endometrial, and nonclinical work on other tumor types

NON-CONFIDENTIAL 2022 KOL Discussion of STRO-002 Phase 1 Interim Dose Expansion Data Q&A Session January 5, 20225:00pm ET / 2:00pm PT