EX-99.1
Published on February 10, 2026

Exhibit 99.1 February 2026 NASDAQ: STRO

Forward-Looking Statements This presentation and the accompanying oral presentation contain “forward-looking” statements that are based on our management’s beliefs and assumptions and on information currently available to management. Forward-looking statements include all statements other than statements of historical fact contained in this presentation, including information concerning our future financial performance; business plans and objectives; anticipated preclinical and clinical development activities, including enrollment and site activation; timing of announcements of clinical results, trial initiation, and regulatory filings; outcome of regulatory decisions; and our expectations about our cash runway; potential benefits of our product candidates and platform; potential expansion into other indications and combinations, including the timing and development activities related to such expansion; potential growth opportunities, financing plans, potential future milestone and royalty payments, competitive position, industry environment and potential market opportunities for our product candidates. Forward-looking statements are subject to known and unknown risks, uncertainties, assumptions and other factors, including risks and uncertainties related to our cash forecasts, our and our collaborators’ ability to advance our product candidates, the receipt, feedback and timing of potential regulatory submissions, designations, approvals and commercialization of product candidates and the design, timing and results of preclinical and clinical trials and our ability to fund development activities and achieve development goals. It is not possible for our management to predict all risks, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements we may make. These factors, together with those that may be described in greater detail under the heading “Risk Factors” contained in our most recent Annual Report on Form 10-K, Quarterly Report on Form 10-Q and other reports the company files from time to time with the Securities and Exchange Commission, may cause our actual results, performance or achievements to differ materially and adversely from those anticipated or implied by our forward-looking statements. You should not rely upon forward-looking statements as predictions of future events. Although our management believes that the expectations reflected in our forward-looking statements are reasonable, we cannot guarantee that the future results, levels of activity, performance or events and circumstances described in the forward-looking statements will be achieved or occur. Moreover, neither we nor our management assume responsibility for the accuracy and completeness of the forward-looking statements. We undertake no obligation to publicly update any forward-looking statements for any reason after the date of this presentation to conform these statements to actual results or to changes in our expectations, except as required by law. This presentation also contains estimates and other statistical data made by independent parties and by us relating to market size and growth and other data about our industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. In addition, projections, assumptions, and estimates of our future performance and the future performance of the markets in which we operate are necessarily subject to a high degree of uncertainty and risk. NON-CONFIDENTIAL 2
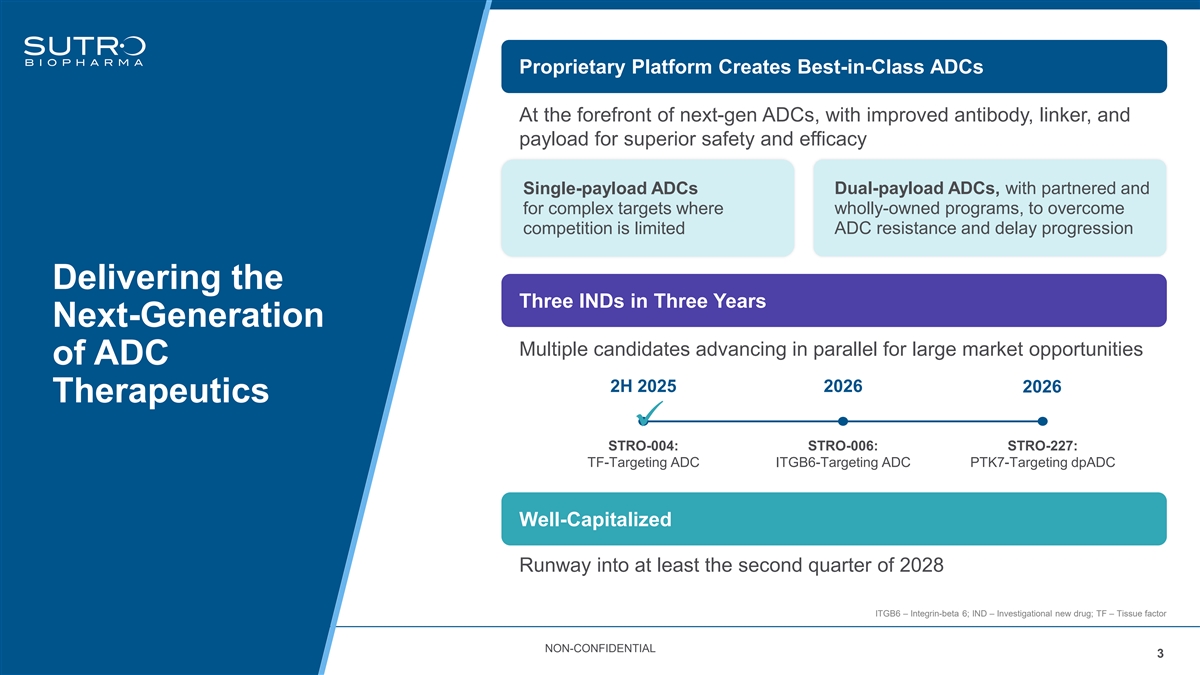
Proprietary Platform Creates Best-in-Class ADCs At the forefront of next-gen ADCs, with improved antibody, linker, and payload for superior safety and efficacy Dual-payload ADCs, with partnered and Single-payload ADCs for complex targets where wholly-owned programs, to overcome competition is limited ADC resistance and delay progression Delivering the Three INDs in Three Years Next-Generation Multiple candidates advancing in parallel for large market opportunities of ADC 2H 2025 2026 2026 Therapeutics ✓ STRO-004: STRO-006: STRO-227: TF-Targeting ADC ITGB6-Targeting ADC PTK7-Targeting dpADC Well-Capitalized Runway into at least the second quarter of 2028 ITGB6 – Integrin-beta 6; IND – Investigational new drug; TF – Tissue factor NON-CONFIDENTIAL 3
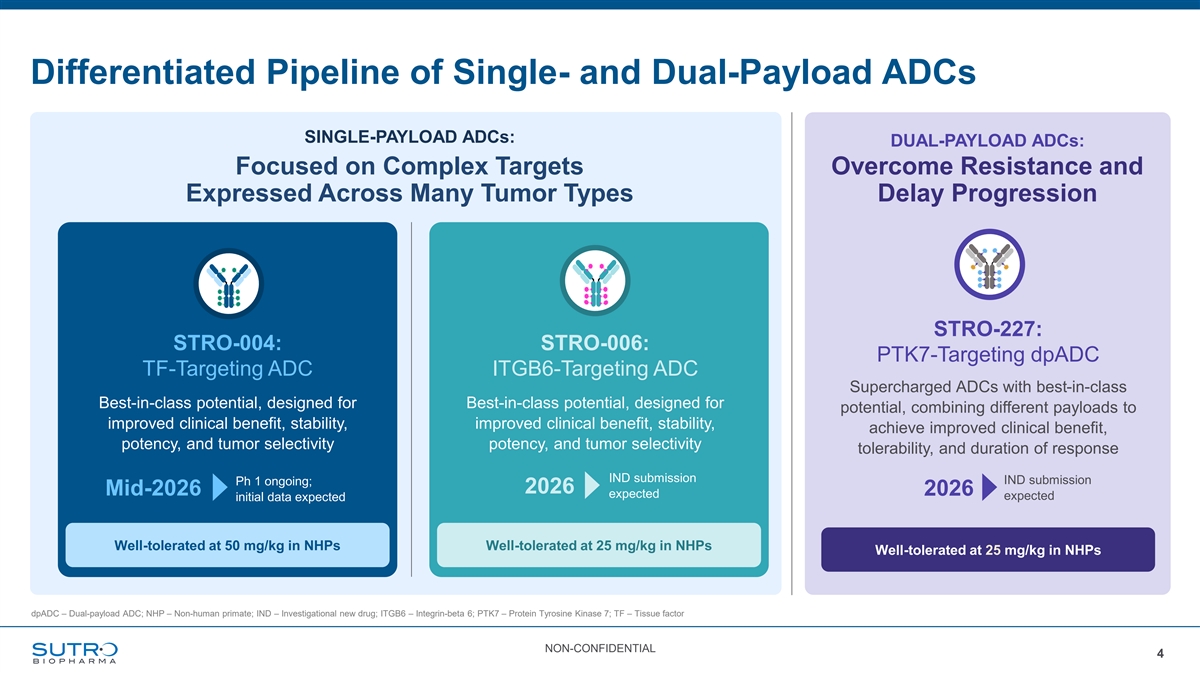
Differentiated Pipeline of Single- and Dual-Payload ADCs SINGLE-PAYLOAD ADCs: DUAL-PAYLOAD ADCs: Focused on Complex Targets Overcome Resistance and Expressed Across Many Tumor Types Delay Progression STRO-227: STRO-004: STRO-006: PTK7-Targeting dpADC TF-Targeting ADC ITGB6-Targeting ADC Supercharged ADCs with best-in-class Best-in-class potential, designed for Best-in-class potential, designed for potential, combining different payloads to improved clinical benefit, stability, improved clinical benefit, stability, achieve improved clinical benefit, potency, and tumor selectivity potency, and tumor selectivity tolerability, and duration of response IND submission IND submission Ph 1 ongoing; 2026 Mid-2026 2026 expected expected initial data expected Well-tolerated at 50 mg/kg in NHPs Well-tolerated at 25 mg/kg in NHPs Well-tolerated at 25 mg/kg in NHPs dpADC – Dual-payload ADC; NHP – Non-human primate; IND – Investigational new drug; ITGB6 – Integrin-beta 6; PTK7 – Protein Tyrosine Kinase 7; TF – Tissue factor NON-CONFIDENTIAL 4

Next-Generation ADCs Enabled by Sutro’s Proprietary Platform
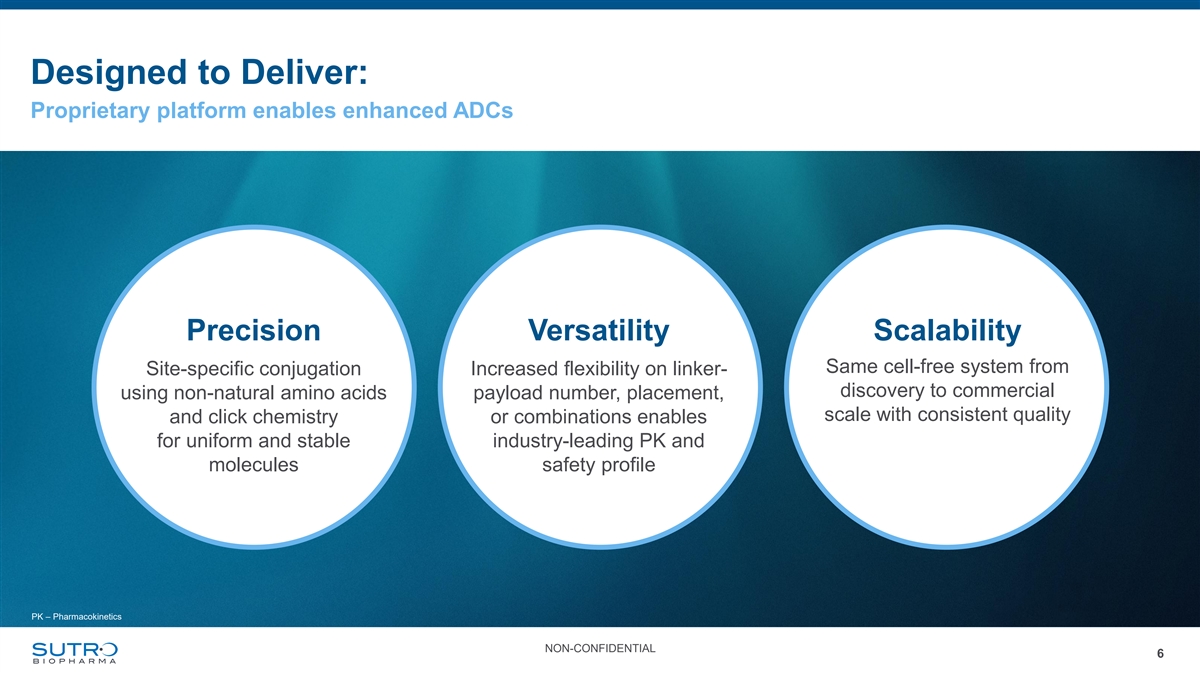
Designed to Deliver: Proprietary platform enables enhanced ADCs Precision Versatility Scalability Same cell-free system from Site-specific conjugation Increased flexibility on linker- discovery to commercial using non-natural amino acids payload number, placement, scale with consistent quality and click chemistry or combinations enables for uniform and stable industry-leading PK and molecules safety profile PK – Pharmacokinetics NON-CONFIDENTIAL 6
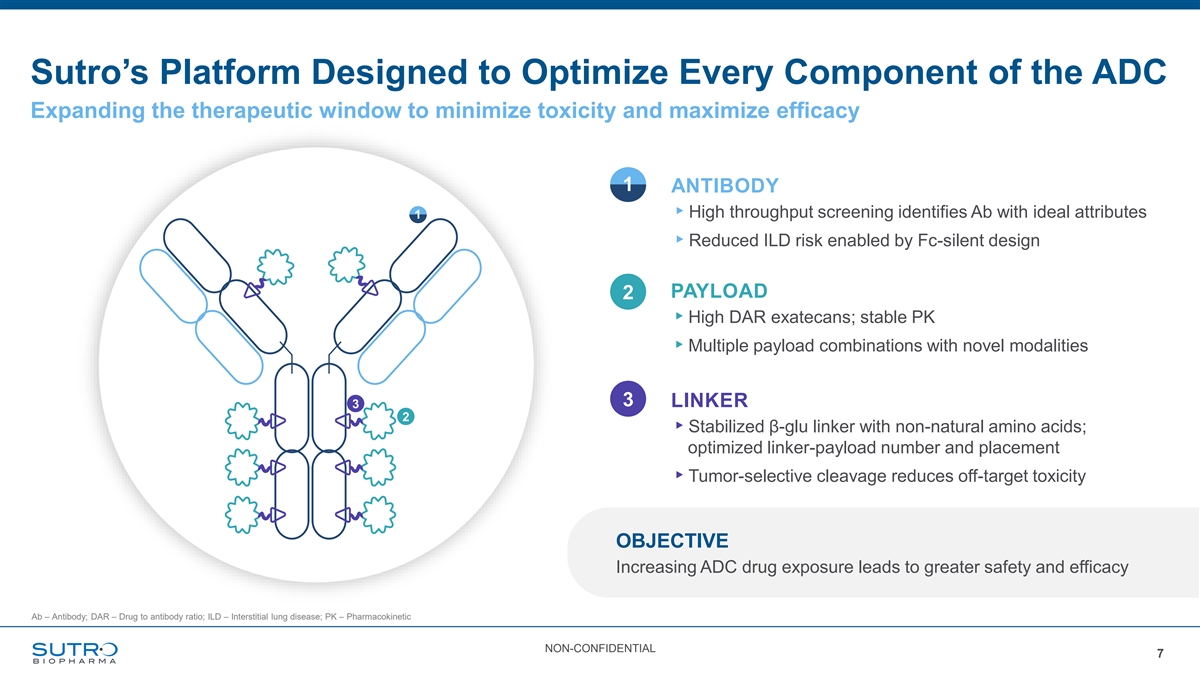
Sutro’s Platform Designed to Optimize Every Component of the ADC Expanding the therapeutic window to minimize toxicity and maximize efficacy 1 ANTIBODY ▸High throughput screening identifies Ab with ideal attributes 1 ▸Reduced ILD risk enabled by Fc-silent design PAYLOAD 2 ▸High DAR exatecans; stable PK ▸Multiple payload combinations with novel modalities 3 LINKER 3 2 ▸Stabilized β-glu linker with non-natural amino acids; optimized linker-payload number and placement ▸Tumor-selective cleavage reduces off-target toxicity OBJECTIVE Increasing ADC drug exposure leads to greater safety and efficacy Ab – Antibody; DAR – Drug to antibody ratio; ILD – Interstitial lung disease; PK – Pharmacokinetic NON-CONFIDENTIAL 7
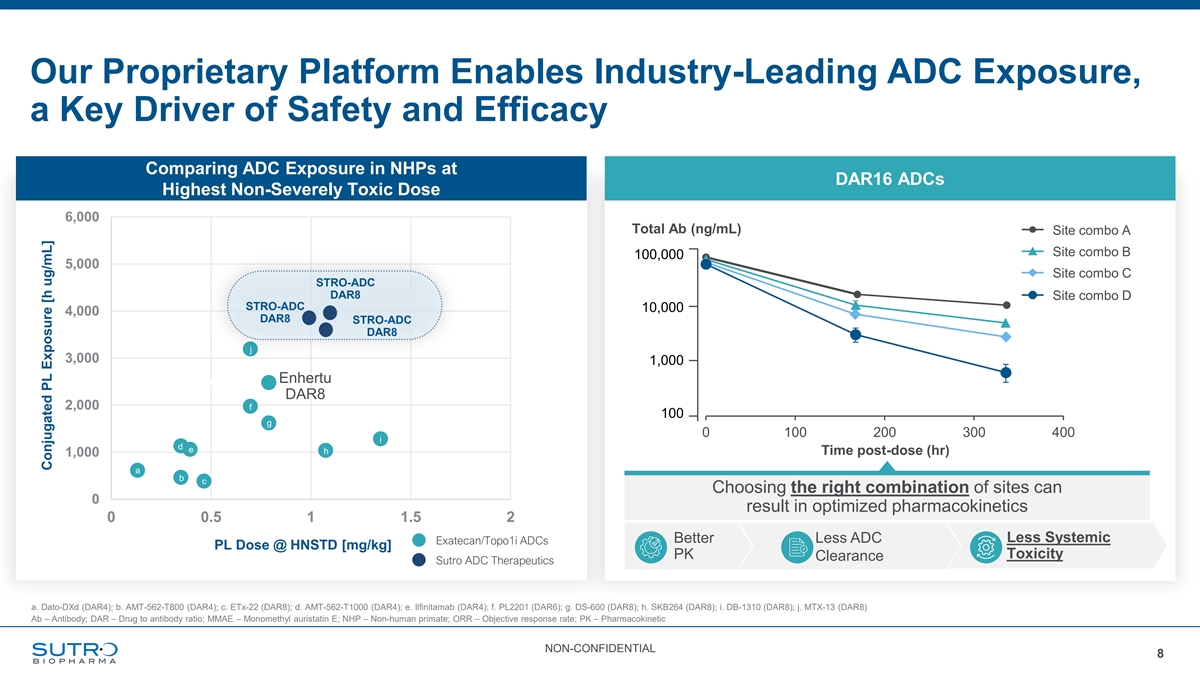
Our Proprietary Platform Enables Industry-Leading ADC Exposure, a Key Driver of Safety and Efficacy Comparing ADC Exposure in NHPs at DAR16 ADCs Highest Non-Severely Toxic Dose 6,000 Total Ab (ng/mL) Site combo A Site combo B 100,000 5,000 Site combo C STRO-ADC DAR8 Site combo D STRO-ADC 10,000 4,000 DAR8 STRO-ADC DAR8 j 3,000 1,000 Enhertu DAR8 2,000 f 100 g 0 100 200 300 400 i d e h Time post-dose (hr) 1,000 a b c Choosing the right combination of sites can 0 result in optimized pharmacokinetics 0 0.5 1 1.5 2 Better Less ADC Less Systemic Exatecan/Topo1i ADCs PL Dose @ HNSTD [mg/kg] PK Toxicity Clearance Sutro ADC Therapeutics a. Dato-DXd (DAR4); b. AMT-562-T800 (DAR4); c. ETx-22 (DAR8); d. AMT-562-T1000 (DAR4); e. Ilfinitamab (DAR4); f. PL2201 (DAR6); g. DS-600 (DAR8); h. SKB264 (DAR8); i. DB-1310 (DAR8); j. MTX-13 (DAR8) Ab – Antibody; DAR – Drug to antibody ratio; MMAE – Monomethyl auristatin E; NHP – Non-human primate; ORR – Objective response rate; PK – Pharmacokinetic NON-CONFIDENTIAL 8 Conjugated PL Exposure [h ug/mL]

STRO-004 Potential Best-in-Class Exatecan ADC Targeting Tissue Factor
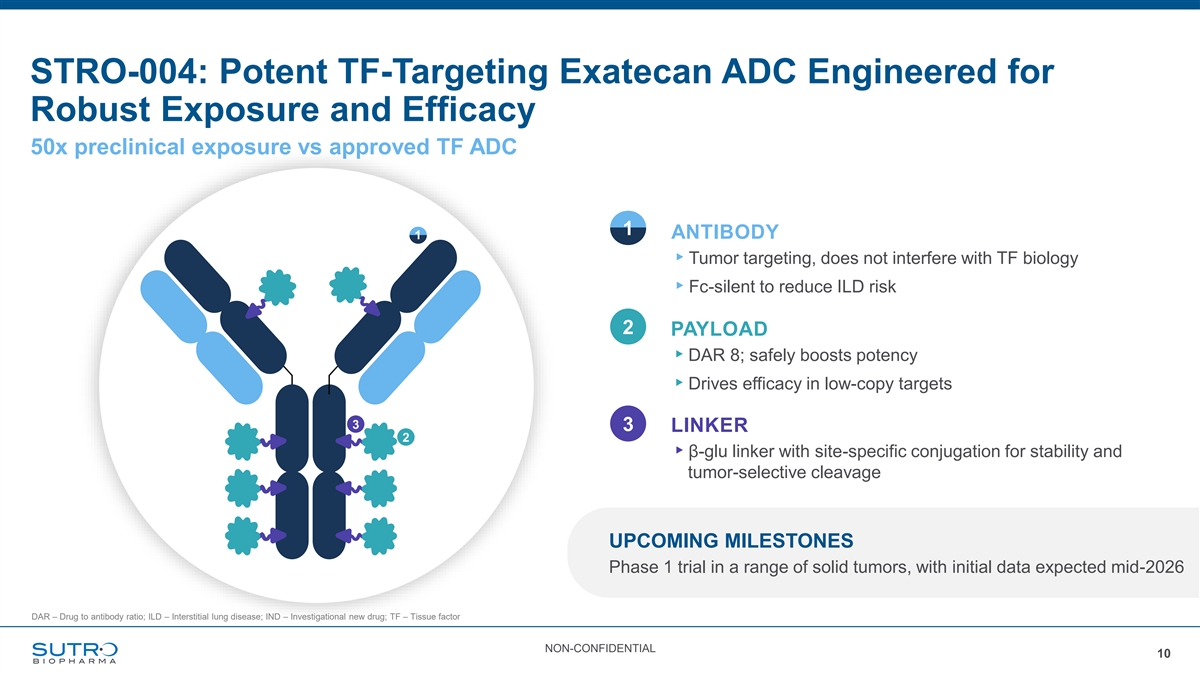
STRO-004: Potent TF-Targeting Exatecan ADC Engineered for Robust Exposure and Efficacy 50x preclinical exposure vs approved TF ADC 1 ANTIBODY 1 ▸Tumor targeting, does not interfere with TF biology ▸Fc-silent to reduce ILD risk 2 PAYLOAD ▸DAR 8; safely boosts potency ▸Drives efficacy in low-copy targets 3 3 LINKER 2 ▸β-glu linker with site-specific conjugation for stability and tumor-selective cleavage UPCOMING MILESTONES Phase 1 trial in a range of solid tumors, with initial data expected mid-2026 DAR – Drug to antibody ratio; ILD – Interstitial lung disease; IND – Investigational new drug; TF – Tissue factor NON-CONFIDENTIAL 10
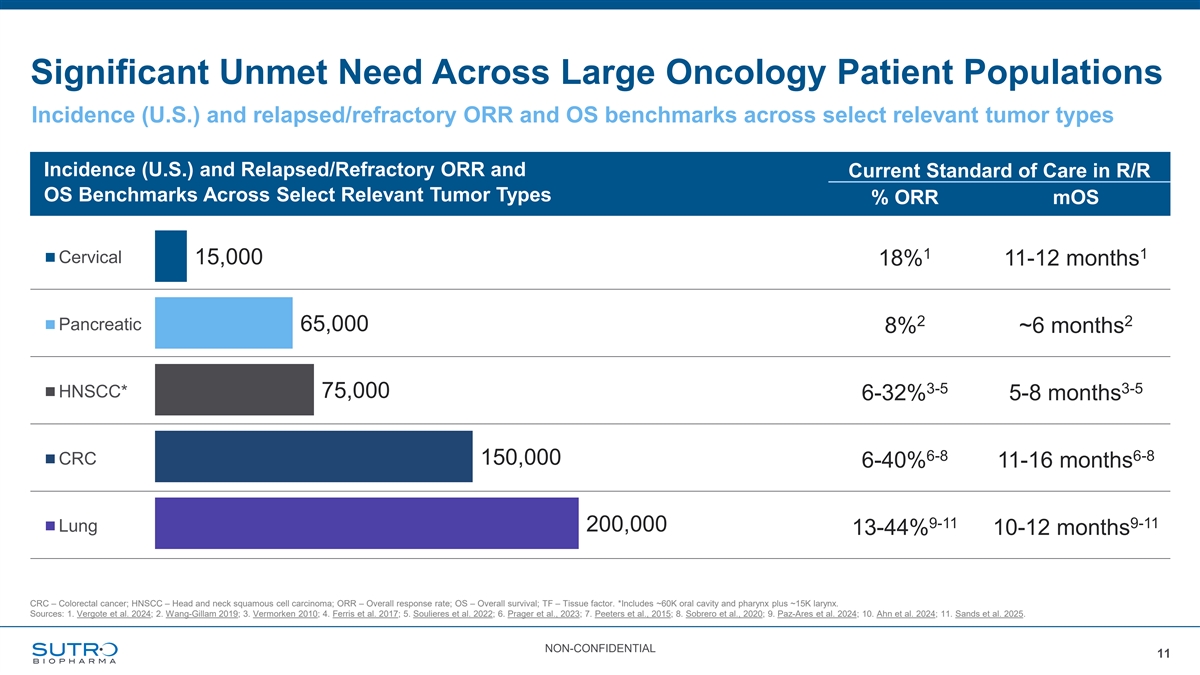
Significant Unmet Need Across Large Oncology Patient Populations Incidence (U.S.) and relapsed/refractory ORR and OS benchmarks across select relevant tumor types Incidence (U.S.) and Relapsed/Refractory ORR and Current Standard of Care in R/R OS Benchmarks Across Select Relevant Tumor Types % ORR mOS 1 1 Cervical 15,000 18% 11-12 months 2 2 Pancreatic 65,000 8% ~6 months 3-5 3-5 HNSCC* 75,000 6-32% 5-8 months 6-8 6-8 CRC 150,000 6-40% 11-16 months 9-11 9-11 200,000 Lung 13-44% 10-12 months CRC – Colorectal cancer; HNSCC – Head and neck squamous cell carcinoma; ORR – Overall response rate; OS – Overall survival; TF – Tissue factor. *Includes ~60K oral cavity and pharynx plus ~15K larynx. Sources: 1. Vergote et al. 2024; 2. Wang-Gillam 2019; 3. Vermorken 2010; 4. Ferris et al. 2017; 5. Soulieres et al. 2022; 6. Prager et al., 2023; 7. Peeters et al., 2015; 8. Sobrero et al., 2020; 9. Paz-Ares et al. 2024; 10. Ahn et al. 2024; 11. Sands et al. 2025. NON-CONFIDENTIAL 11
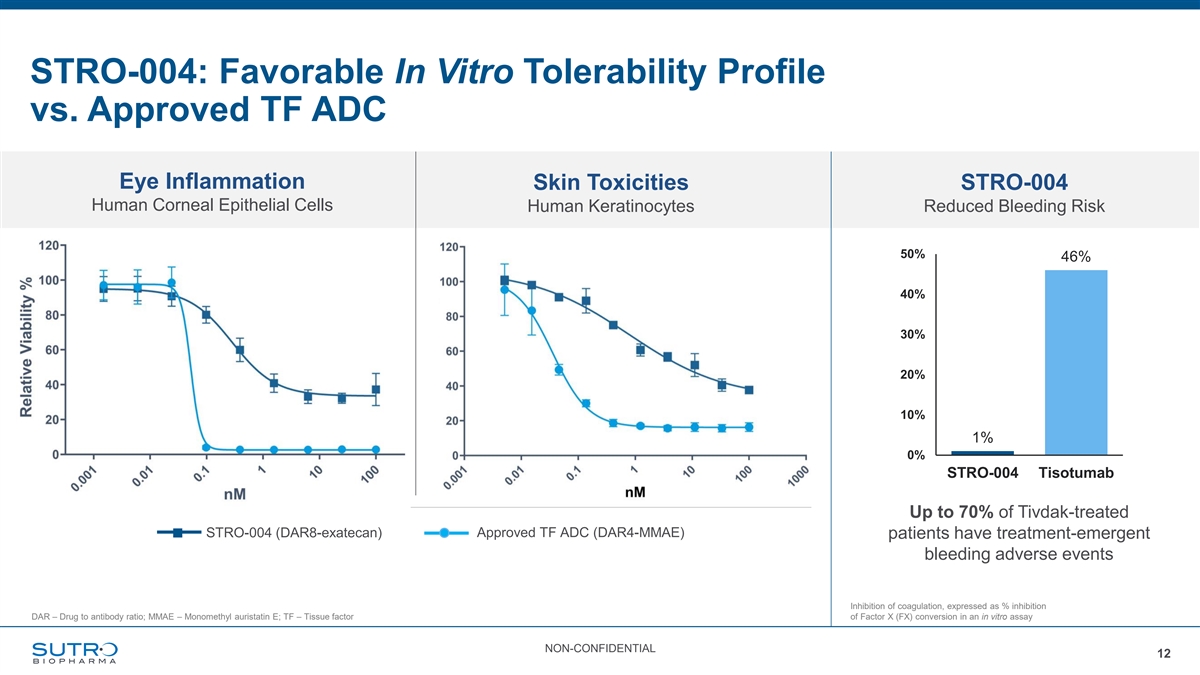
STRO-004: Favorable In Vitro Tolerability Profile vs. Approved TF ADC Eye Inflammation Skin Toxicities STRO-004 Human Corneal Epithelial Cells Human Keratinocytes Reduced Bleeding Risk 50% 46% 40% 30% 20% 10% 1% 0% STRO-004 Tisotumab Up to 70% of Tivdak-treated STRO-004 (DAR8-exatecan) Approved TF ADC (DAR4-MMAE) patients have treatment-emergent bleeding adverse events Inhibition of coagulation, expressed as % inhibition DAR – Drug to antibody ratio; MMAE – Monomethyl auristatin E; TF – Tissue factor of Factor X (FX) conversion in an in vitro assay NON-CONFIDENTIAL 12
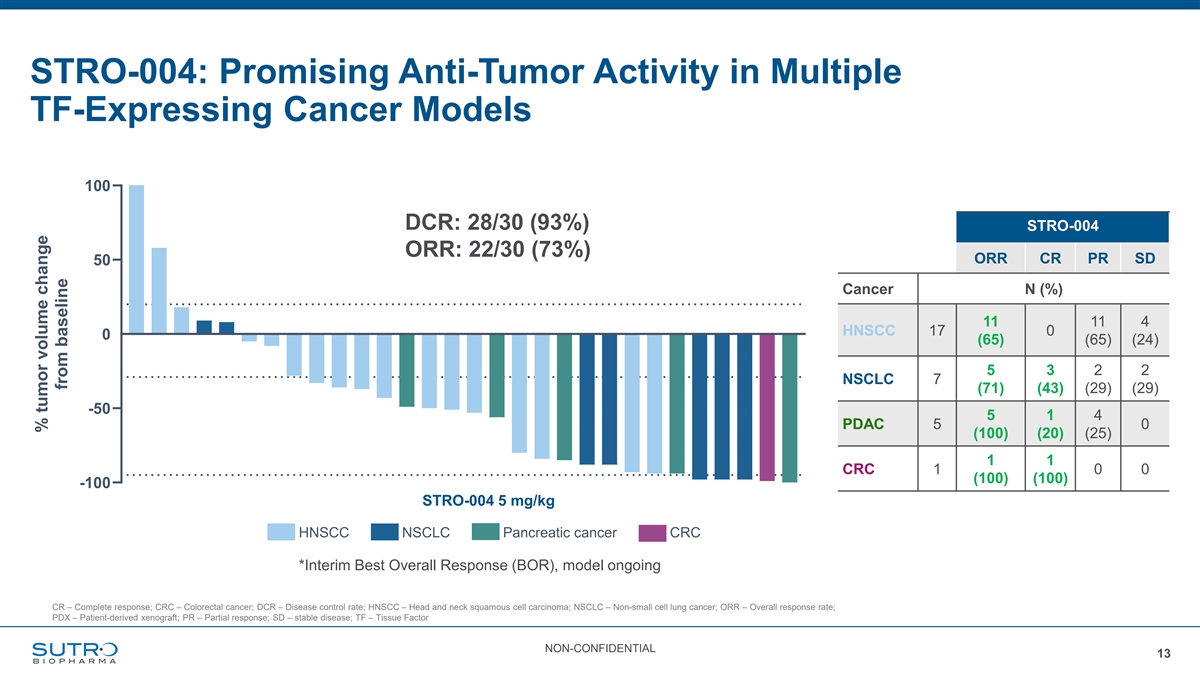
STRO-004: Promising Anti-Tumor Activity in Multiple TF-Expressing Cancer Models STRO-004 % Best Response in PDX Models of Solid Tumors 100 DCR: 28/30 ORR: 22/30 DCR: 28/30 (93%) STRO-004 ORR: 22/30 (73%) ORR CR PR SD 50 Cancer N (%) 11 11 4 HNSCC 17 0 0 (65) (65) (24) 5 3 2 2 NSCLC 7 (71) (43) (29) (29) -50 5 1 4 PDAC 5 0 (100) (20) (25) 1 1 CRC 1 0 0 (100) (100) -100 STRO-004 5 mg/kg HNSCC NSCLC Pancreatic cancer CRC *Interim Best Overall Response (BOR), model ongoing CR – Complete response; CRC – Colorectal cancer; DCR – Disease control rate; HNSCC – Head and neck squamous cell carcinoma; NSCLC – Non-small cell lung cancer; ORR – Overall response rate; PDX – Patient-derived xenograft; PR – Partial response; SD – stable disease; TF – Tissue Factor NON-CONFIDENTIAL 13 % tumor volume change from baseline
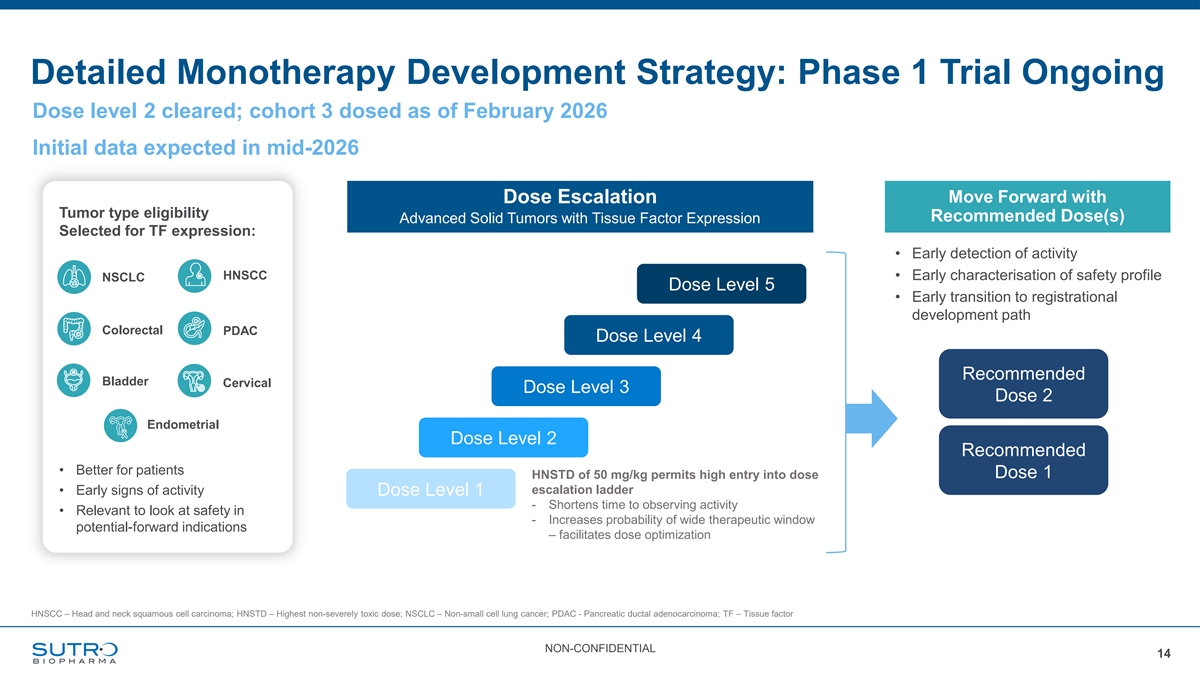
Detailed Monotherapy Development Strategy: Phase 1 Trial Ongoing Dose level 2 cleared; cohort 3 dosed as of February 2026 Initial data expected in mid-2026 Move Forward with Dose Escalation Tumor type eligibility Recommended Dose(s) Advanced Solid Tumors with Tissue Factor Expression Selected for TF expression: • Early detection of activity HNSCC • Early characterisation of safety profile NSCLC Dose Level 5 • Early transition to registrational development path Colorectal PDAC Dose Level 4 Recommended Bladder Cervical Dose Level 3 Dose 2 Endometrial Dose Level 2 Recommended • Better for patients HNSTD of 50 mg/kg permits high entry into dose Dose 1 escalation ladder • Early signs of activity Dose Level 1 - Shortens time to observing activity • Relevant to look at safety in - Increases probability of wide therapeutic window potential-forward indications – facilitates dose optimization HNSCC – Head and neck squamous cell carcinoma; HNSTD – Highest non-severely toxic dose; NSCLC – Non-small cell lung cancer; PDAC - Pancreatic ductal adenocarcinoma; TF – Tissue factor NON-CONFIDENTIAL 14

STRO-006 Potential Best-in-Class Exatecan ADC Targeting Integrin-Beta 6
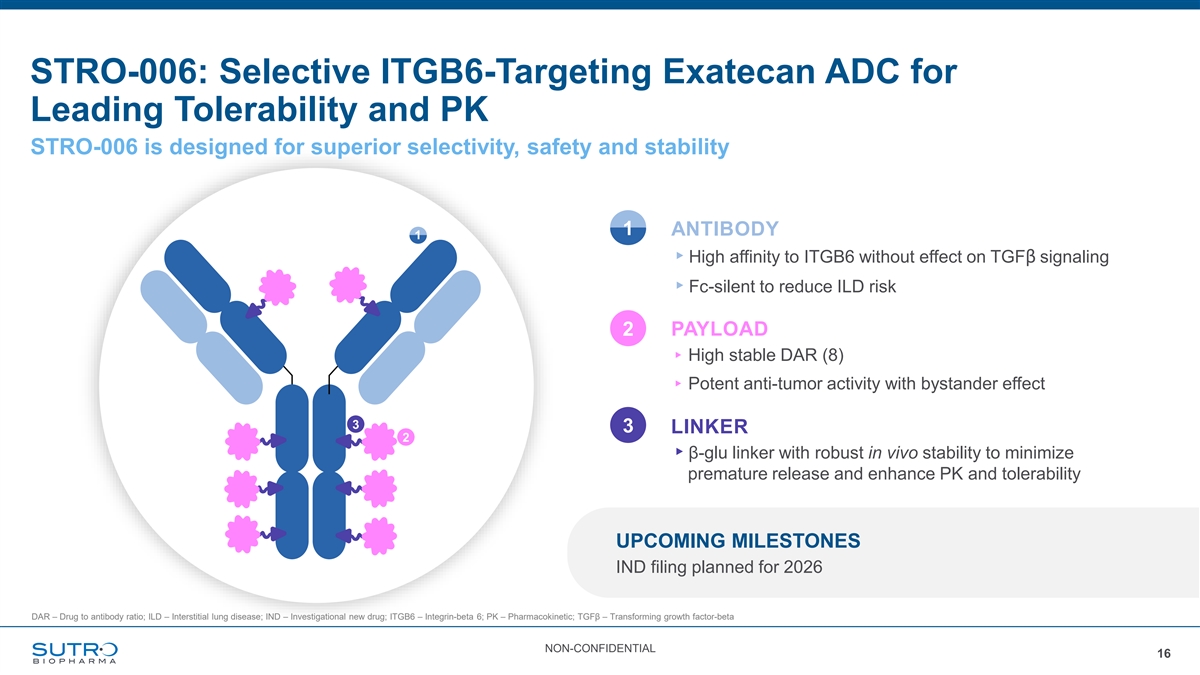
STRO-006: Selective ITGB6-Targeting Exatecan ADC for Leading Tolerability and PK STRO-006 is designed for superior selectivity, safety and stability 1 ANTIBODY 1 ▸High affinity to ITGB6 without effect on TGFβ signaling ▸Fc-silent to reduce ILD risk 2 PAYLOAD ▸ High stable DAR (8) ▸ Potent anti-tumor activity with bystander effect 3 3 LINKER 2 ▸β-glu linker with robust in vivo stability to minimize premature release and enhance PK and tolerability UPCOMING MILESTONES IND filing planned for 2026 DAR – Drug to antibody ratio; ILD – Interstitial lung disease; IND – Investigational new drug; ITGB6 – Integrin-beta 6; PK – Pharmacokinetic; TGFβ – Transforming growth factor-beta NON-CONFIDENTIAL 16
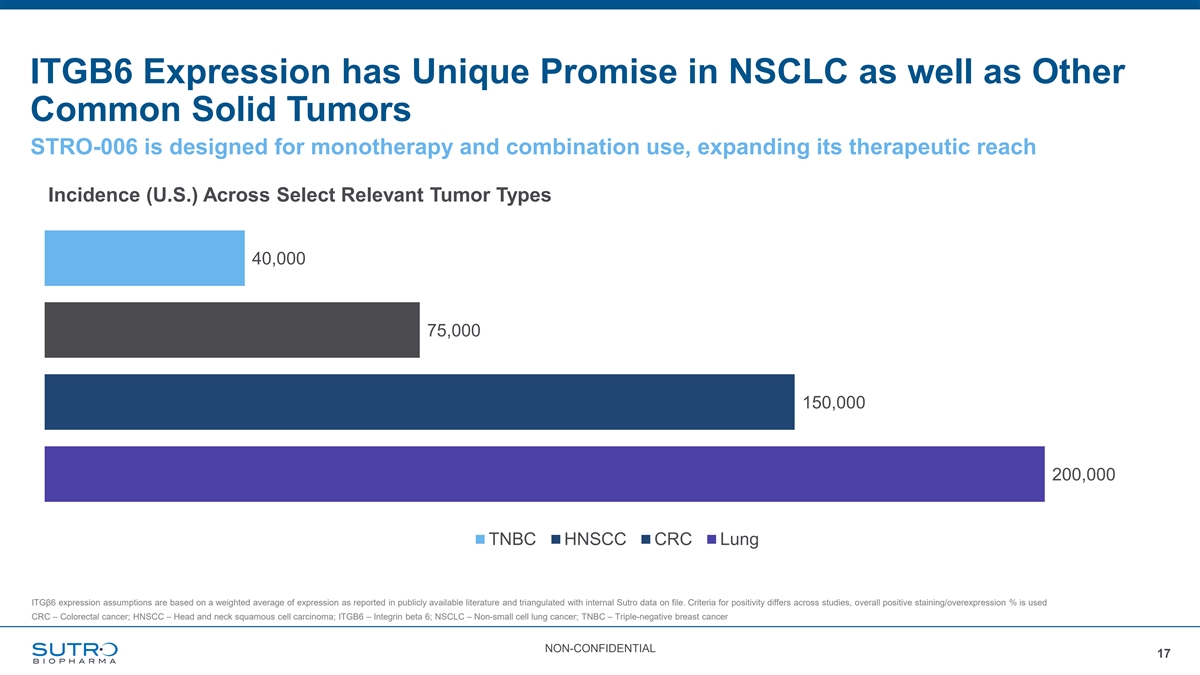
ITGB6 Expression has Unique Promise in NSCLC as well as Other Common Solid Tumors STRO-006 is designed for monotherapy and combination use, expanding its therapeutic reach Incidence (U.S.) Across Select Relevant Tumor Types 40,000 75,000 150,000 200,000 TNBC HNSCC CRC Lung ITGβ6 expression assumptions are based on a weighted average of expression as reported in publicly available literature and triangulated with internal Sutro data on file. Criteria for positivity differs across studies, overall positive staining/overexpression % is used CRC – Colorectal cancer; HNSCC – Head and neck squamous cell carcinoma; ITGB6 – Integrin beta 6; NSCLC – Non-small cell lung cancer; TNBC – Triple-negative breast cancer NON-CONFIDENTIAL 17
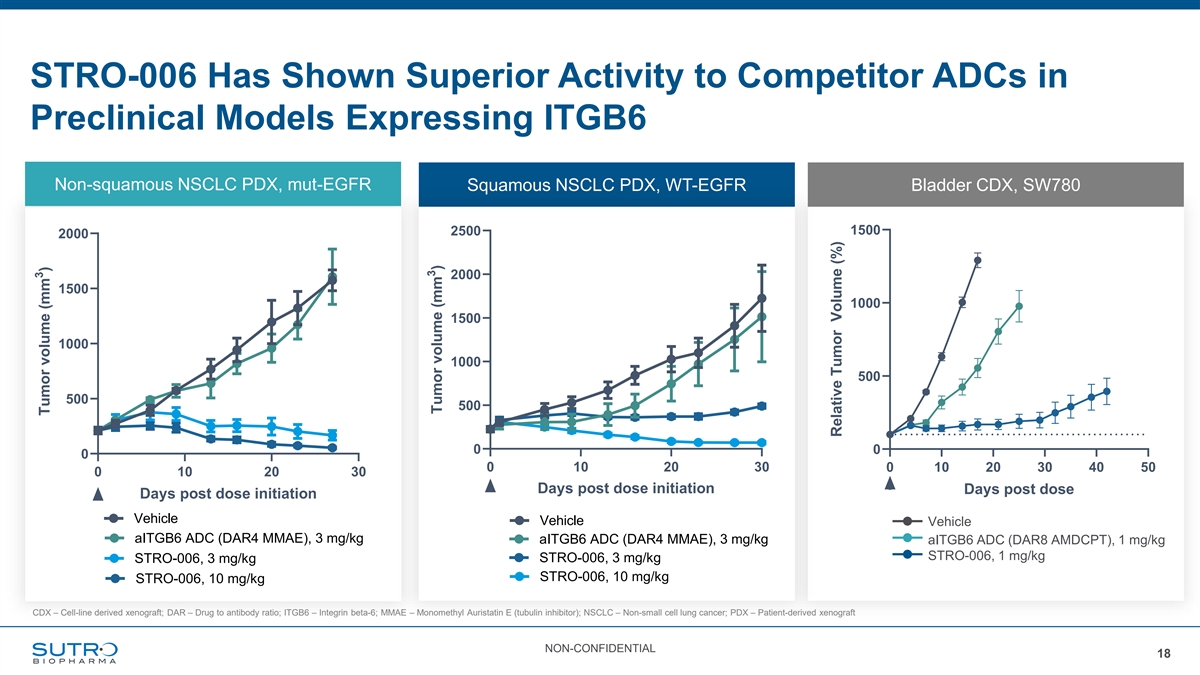
STRO-006 Has Shown Superior Activity to Competitor ADCs in Preclinical Models Expressing ITGB6 adeno NSCLC PDX (CTG-0743) Non-squamous NSCLC PDX, mut-EGFR Squamous NSCLC PDX, WT-EGFR Bladder CDX, SW780 H score: 136 (Seagen) SW780 (UCC) 1500 2500 2000 ITGB6-moderate 2000 1500 1000 1500 1000 1000 500 500 500 0 0 0 0 10 20 30 0 10 20 30 40 50 0 10 20 30 Days post dose initiation Days post dose Days post dose initiation Vehicle Vehicle Vehicle aITGB6 ADC (DAR4 MMAE), 3 mg/kg aITGB6 ADC (DAR4 MMAE), 3 mg/kg aITGB6 ADC (DAR8 AMDCPT), 1 mg/kg STRO-006, 1 mg/kg STRO-006, 3 mg/kg STRO-006, 3 mg/kg STRO-006, 10 mg/kg STRO-006, 10 mg/kg CDX – Cell-line derived xenograft; DAR – Drug to antibody ratio; ITGB6 – Integrin beta-6; MMAE – Monomethyl Auristatin E (tubulin inhibitor); NSCLC – Non-small cell lung cancer; PDX – Patient-derived xenograft NON-CONFIDENTIAL 18 3 Tumor volume (mm ) 3 Tumor volume (mm ) Relative Tumor Volume (%)
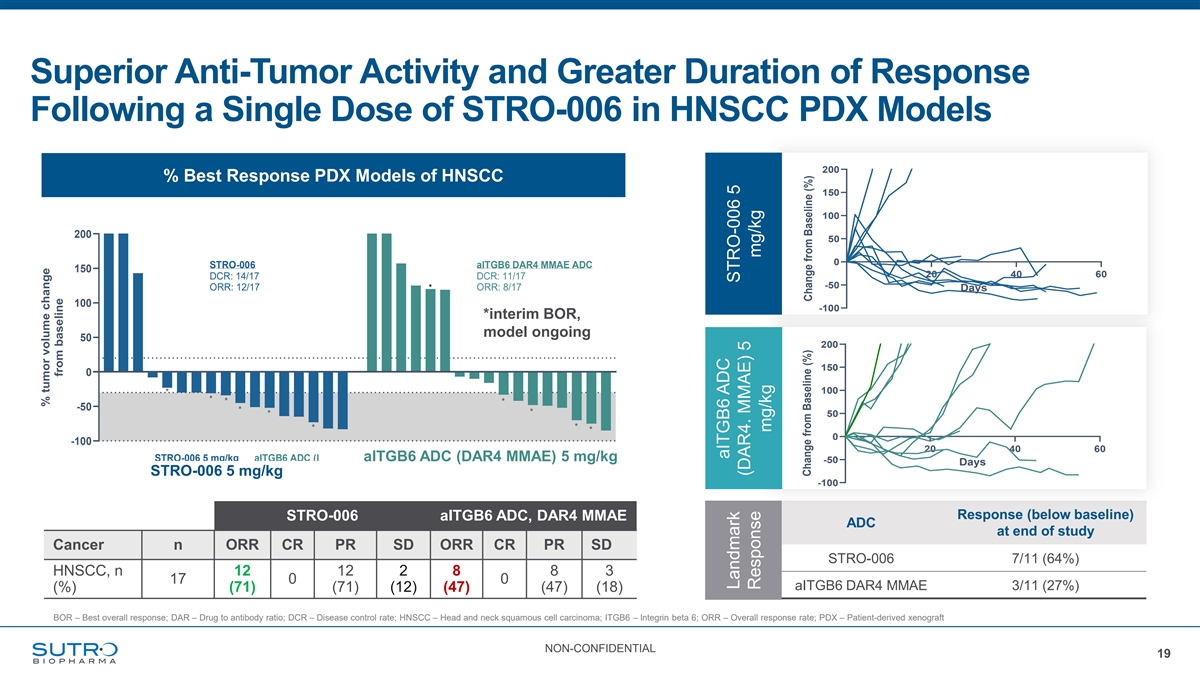
Superior Anti-Tumor Activity and Greater Duration of Response Following a Single Dose of STRO-006 in HNSCC PDX Models 200 % Best Response PDX Models of HNSCC 150 100 200 50 % Best Response in PDX Models of HNSCC 0 STRO-006 aITGB6 DAR4 MMAE ADC 150 20 40 60 DCR: 14/17 DCR: 11/17 -50 ORR: 12/17 * ORR: 8/17 Days 100 -100 *interim BOR, model ongoing 50 200 19 150 0 100 * * * * -50 * * * 50 * * * 0 -100 20 40 60 *: interim readout, model ongoing STRO-006 5 mg/kg aITGB6 ADC (DAR4 MMAa ElTGB6 ) 5 mg/kg ADC (DAR4 MMAE) 5 mg/kg -50 Days STRO-006 5 mg/kg -100 Response (below baseline) STRO-006 aITGB6 ADC, DAR4 MMAE ADC at end of study Cancer n ORR CR PR SD ORR CR PR SD STRO-006 7/11 (64%) HNSCC, n 12 12 2 8 8 3 17 0 0 aITGB6 DAR4 MMAE 3/11 (27%) (%) (71) (71) (12) (47) (47) (18) BOR – Best overall response; DAR – Drug to antibody ratio; DCR – Disease control rate; HNSCC – Head and neck squamous cell carcinoma; ITGB6 – Integrin beta 6; ORR – Overall response rate; PDX – Patient-derived xenograft NON-CONFIDENTIAL 19 % tumor volume change from baseline aITGB6 ADC Landmark STRO-006 5 (DAR4. MMAE) 5 Response mg/kg mg/kg Change from Baseline (%) Change from Baseline (%)

Delivering Dual-Payloads: The Next Revolution in ADCs
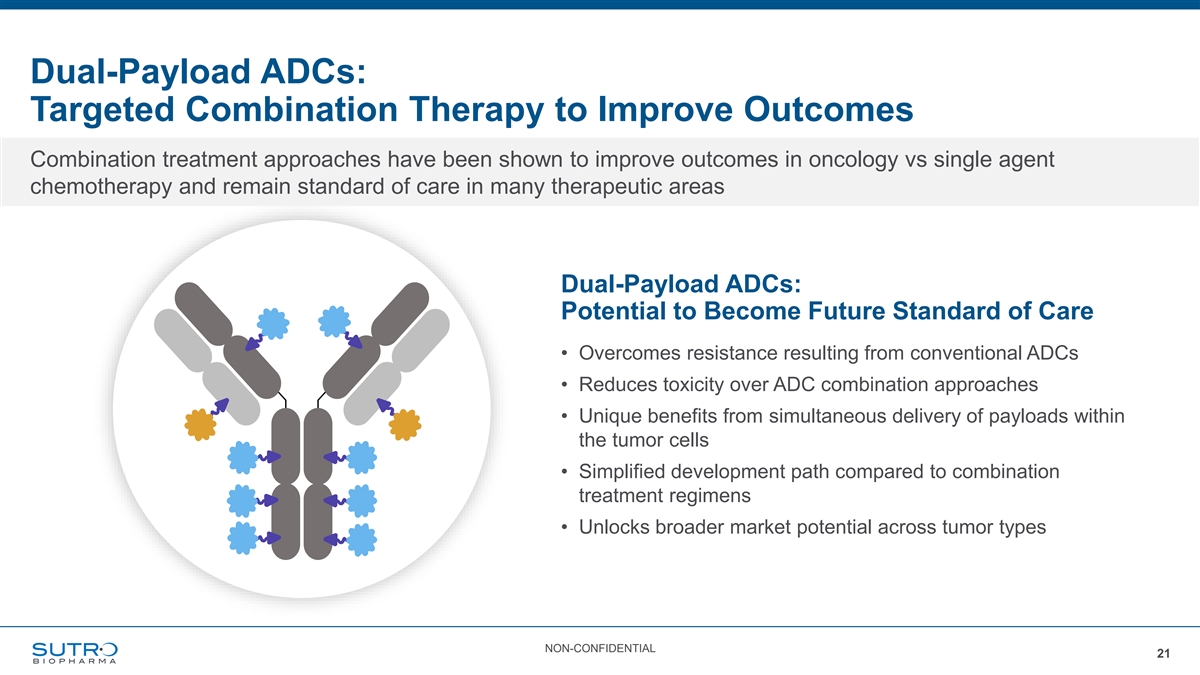
Dual-Payload ADCs: Targeted Combination Therapy to Improve Outcomes Combination treatment approaches have been shown to improve outcomes in oncology vs single agent chemotherapy and remain standard of care in many therapeutic areas Dual-Payload ADCs: Potential to Become Future Standard of Care • Overcomes resistance resulting from conventional ADCs • Reduces toxicity over ADC combination approaches • Unique benefits from simultaneous delivery of payloads within the tumor cells • Simplified development path compared to combination treatment regimens • Unlocks broader market potential across tumor types NON-CONFIDENTIAL 21
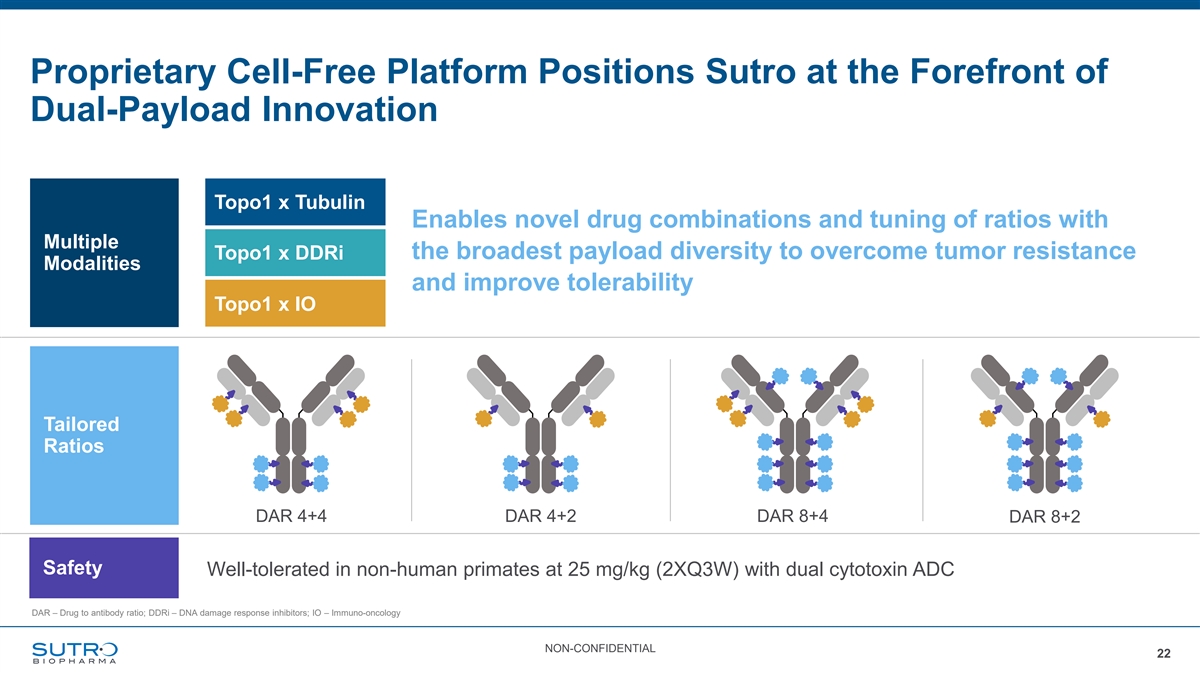
Proprietary Cell-Free Platform Positions Sutro at the Forefront of Dual-Payload Innovation Topo1 x Tubulin Enables novel drug combinations and tuning of ratios with Multiple the broadest payload diversity to overcome tumor resistance Topo1 x DDRi Modalities and improve tolerability Topo1 x IO Tailored Ratios DAR 4+4 DAR 4+2 DAR 8+4 DAR 8+2 Safety Well-tolerated in non-human primates at 25 mg/kg (2XQ3W) with dual cytotoxin ADC DAR – Drug to antibody ratio; DDRi – DNA damage response inhibitors; IO – Immuno-oncology NON-CONFIDENTIAL 22
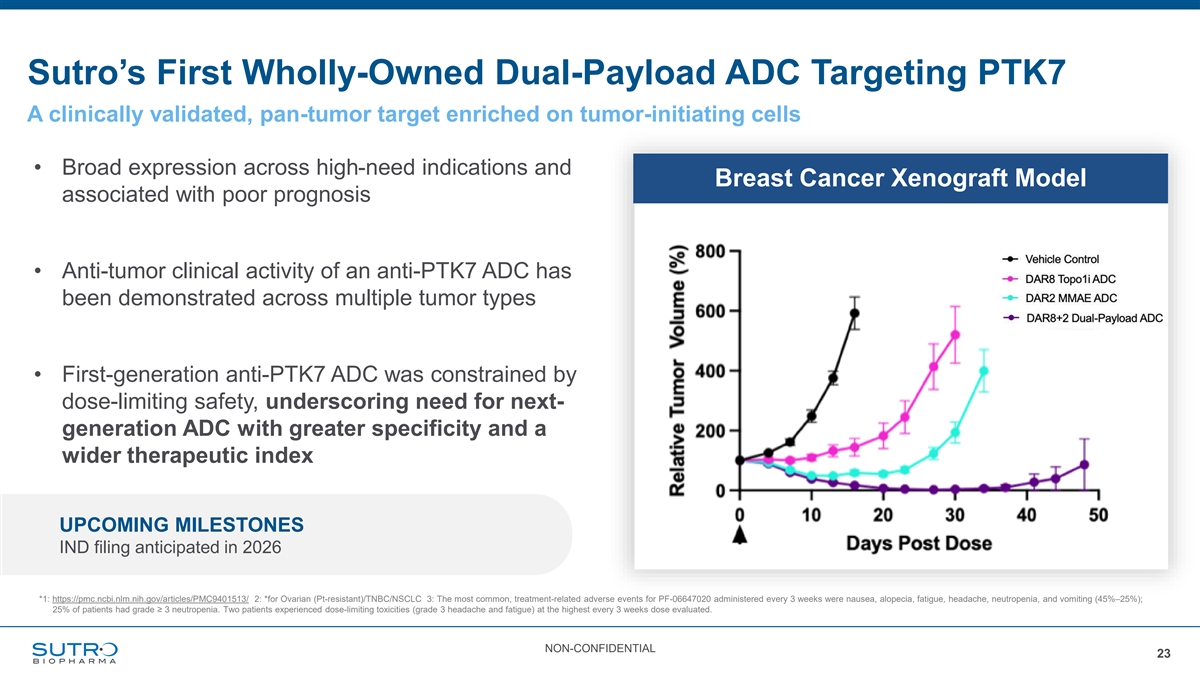
Sutro’s First Wholly-Owned Dual-Payload ADC Targeting PTK7 A clinically validated, pan-tumor target enriched on tumor-initiating cells • Broad expression across high-need indications and Breast Cancer Xenograft Model associated with poor prognosis • Anti-tumor clinical activity of an anti-PTK7 ADC has been demonstrated across multiple tumor types • First-generation anti-PTK7 ADC was constrained by dose-limiting safety, underscoring need for next- generation ADC with greater specificity and a wider therapeutic index UPCOMING MILESTONES IND filing anticipated in 2026 *1: https://pmc.ncbi.nlm.nih.gov/articles/PMC9401513/ 2: *for Ovarian (Pt-resistant)/TNBC/NSCLC 3: The most common, treatment-related adverse events for PF-06647020 administered every 3 weeks were nausea, alopecia, fatigue, headache, neutropenia, and vomiting (45%–25%); 25% of patients had grade ≥ 3 neutropenia. Two patients experienced dose-limiting toxicities (grade 3 headache and fatigue) at the highest every 3 weeks dose evaluated. NON-CONFIDENTIAL 23
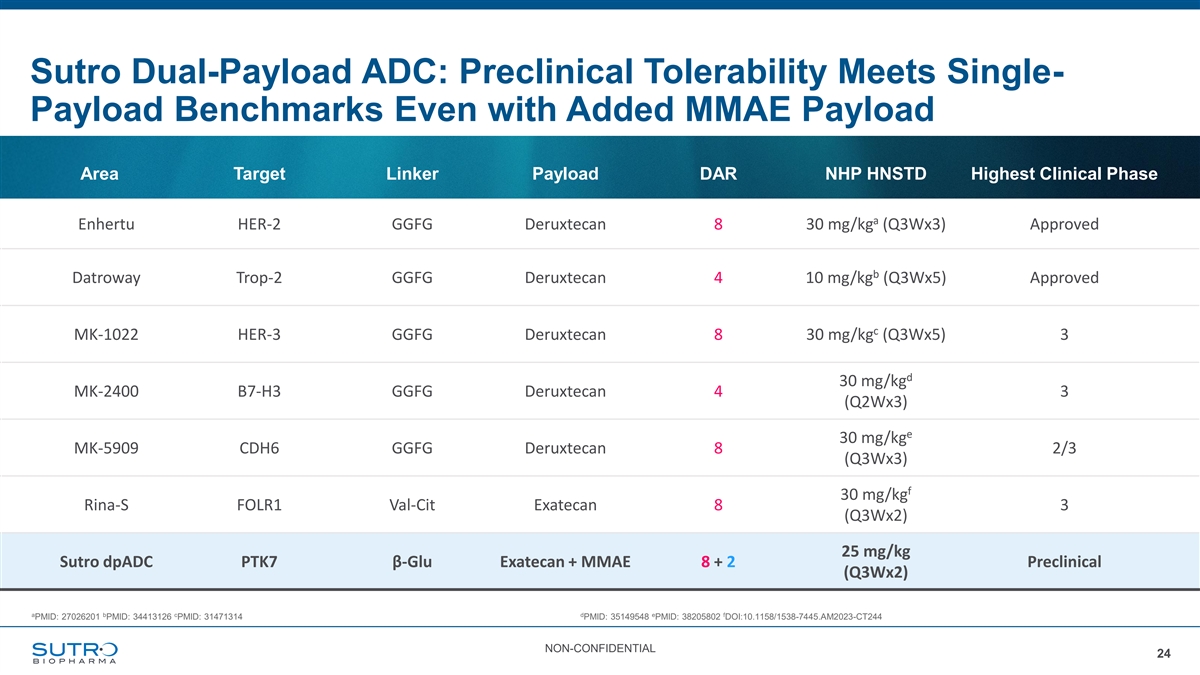
Sutro Dual-Payload ADC: Preclinical Tolerability Meets Single- Payload Benchmarks Even with Added MMAE Payload Area Target Linker Payload DAR NHP HNSTD Highest Clinical Phase a Enhertu HER-2 GGFG Deruxtecan 8 30 mg/kg (Q3Wx3) Approved b Datroway Trop-2 GGFG Deruxtecan 4 10 mg/kg (Q3Wx5) Approved c MK-1022 HER-3 GGFG Deruxtecan 8 30 mg/kg (Q3Wx5) 3 d 30 mg/kg MK-2400 B7-H3 GGFG Deruxtecan 4 3 (Q2Wx3) e 30 mg/kg MK-5909 CDH6 GGFG Deruxtecan 8 2/3 (Q3Wx3) f 30 mg/kg Rina-S FOLR1 Val-Cit Exatecan 8 3 (Q3Wx2) 25 mg/kg Sutro dpADC PTK7β-Glu Exatecan + MMAE 8 + 2 Preclinical (Q3Wx2) a b c d e f PMID: 27026201 PMID: 34413126 PMID: 31471314 PMID: 35149548 PMID: 38205802 DOI:10.1158/1538-7445.AM2023-CT244 NON-CONFIDENTIAL 24
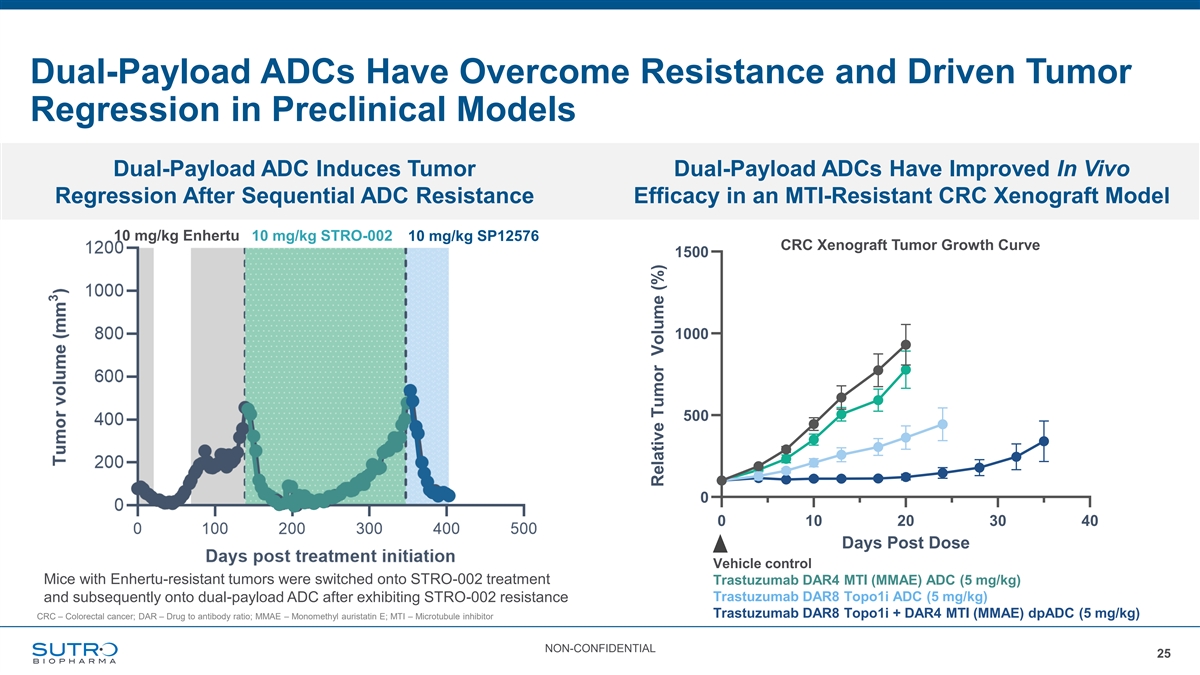
Dual-Payload ADCs Have Overcome Resistance and Driven Tumor Regression in Preclinical Models Dual-Payload ADC Induces Tumor Dual-Payload ADCs Have Improved In Vivo Regression After Sequential ADC Resistance Efficacy in an MTI-Resistant CRC Xenograft Model HCT-116 Tumor Growth Curves 10 mg/kg Enhertu 10 mg/kg STRO-002 10 mg/kg SP12576 CRC Xenograft Tumor Growth Curve 1500 1000 500 0 0 10 20 30 40 Days Post Dose Vehicle control Mice with Enhertu-resistant tumors were switched onto STRO-002 treatment Trastuzumab DAR4 MTI (MMAE) ADC (5 mg/kg) Trastuzumab DAR8 Topo1i ADC (5 mg/kg) and subsequently onto dual-payload ADC after exhibiting STRO-002 resistance Trastuzumab DAR8 Topo1i + DAR4 MTI (MMAE) dpADC (5 mg/kg) CRC – Colorectal cancer; DAR – Drug to antibody ratio; MMAE – Monomethyl auristatin E; MTI – Microtubule inhibitor NON-CONFIDENTIAL 25 Relative Tumor Volume (%)
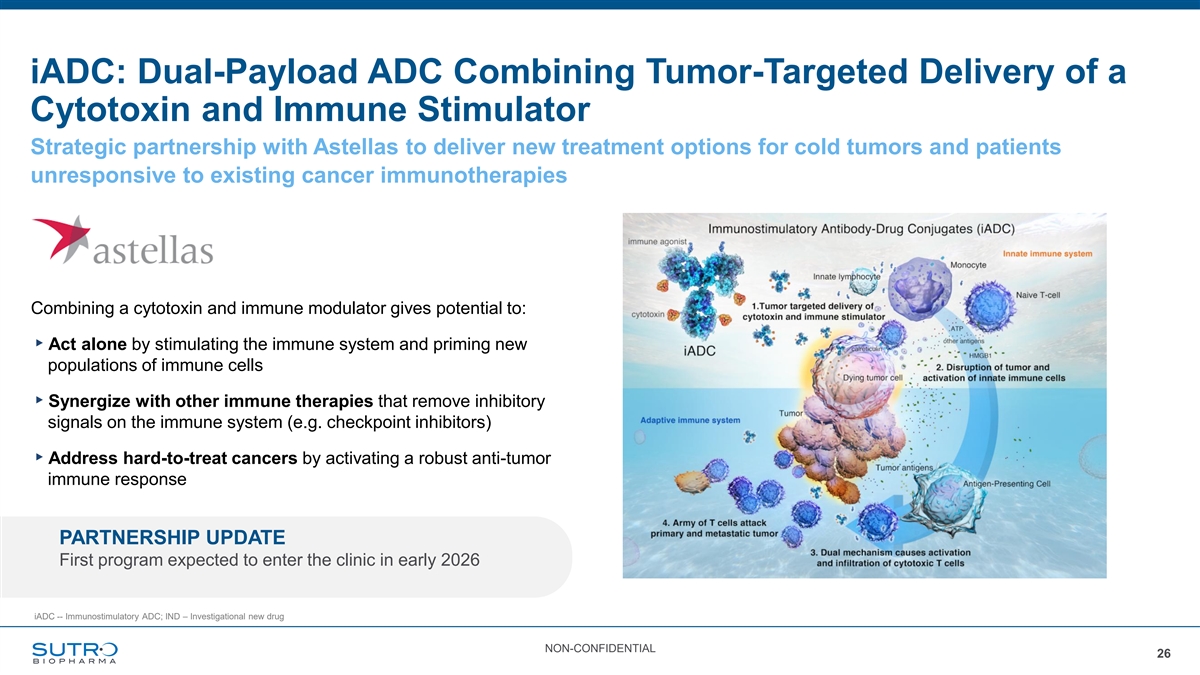
iADC: Dual-Payload ADC Combining Tumor-Targeted Delivery of a Cytotoxin and Immune Stimulator Strategic partnership with Astellas to deliver new treatment options for cold tumors and patients unresponsive to existing cancer immunotherapies Combining a cytotoxin and immune modulator gives potential to: ▸Act alone by stimulating the immune system and priming new populations of immune cells ▸Synergize with other immune therapies that remove inhibitory signals on the immune system (e.g. checkpoint inhibitors) ▸Address hard-to-treat cancers by activating a robust anti-tumor immune response PARTNERSHIP UPDATE First program expected to enter the clinic in early 2026 iADC -- Immunostimulatory ADC; IND – Investigational new drug NON-CONFIDENTIAL 26
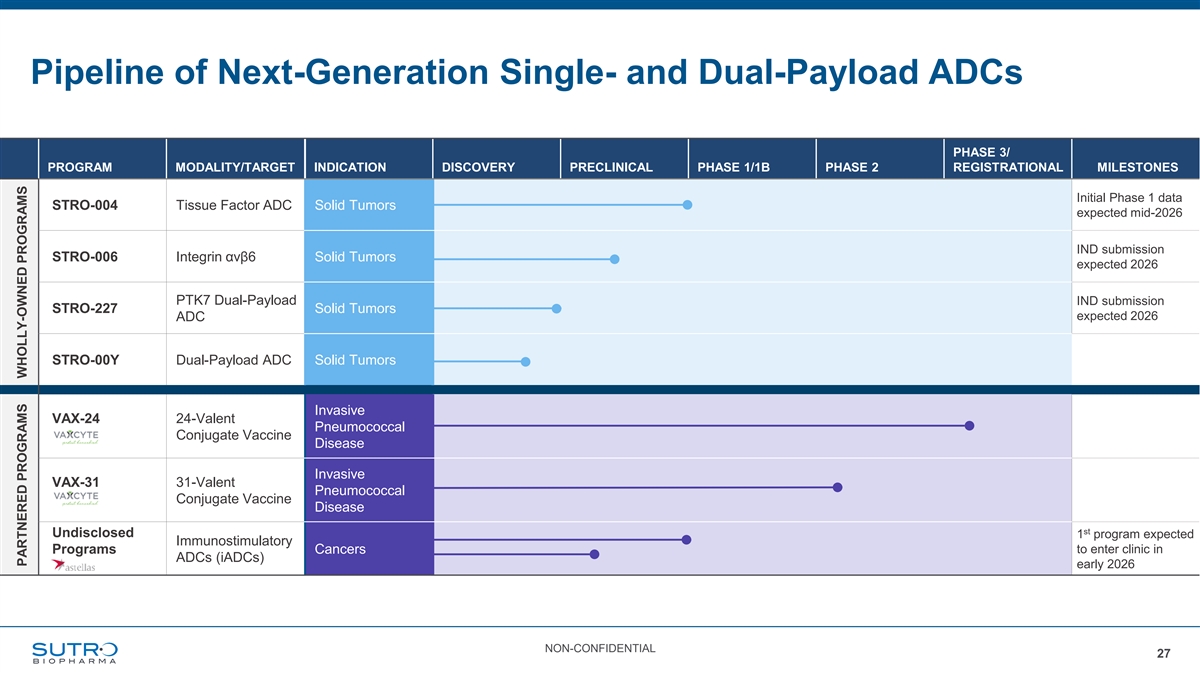
Pipeline of Next-Generation Single- and Dual-Payload ADCs PHASE 3/ PROGRAM MODALITY/TARGET INDICATION DISCOVERY PRECLINICAL PHASE 1/1B PHASE 2 REGISTRATIONAL MILESTONES Initial Phase 1 data STRO-004 Tissue Factor ADC Solid Tumors expected mid-2026 IND submission STRO-006 Integrin αvβ6 Solid Tumors expected 2026 PTK7 Dual-Payload IND submission STRO-227 Solid Tumors expected 2026 ADC STRO-00Y Dual-Payload ADC Solid Tumors Invasive VAX-24 24-Valent Pneumococcal Conjugate Vaccine Disease Invasive VAX-31 31-Valent Pneumococcal Conjugate Vaccine Disease st Undisclosed 1 program expected Immunostimulatory Programs Cancers to enter clinic in ADCs (iADCs) early 2026 NON-CONFIDENTIAL 27 PARTNERED PROGRAMS WHOLLY-OWNED PROGRAMS