DEF 14A: Definitive proxy statements
Published on April 26, 2024
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
Proxy Statement Pursuant to Section 14(a) of the
Securities Exchange Act of 1934
(AMENDMENT NO. )
Filed by the Registrant ☒ Filed by a Party other than the Registrant ☐
Check the appropriate box:
☐ Preliminary Proxy Statement
☐ Confidential, for Use of the Commission Only (as permitted by Rule 14a-6(e)(2))
☒ Definitive Proxy Statement
☐ Definitive Additional Materials
☐ Soliciting Material Pursuant to §240.14a-12
(Name of Registrant as Specified In Its Charter)
N/A
(Name of Person(s) Filing Proxy Statement, if other than the Registrant)
Payment of Filing Fee (Check the appropriate box):
☒ No fee required.
☐ Fee paid previously with preliminary materials.
☐ Fee computed on table in exhibit required by Item 25(b) per Exchange Act Rules 14a-6(i)(1) and 0-11.
SUTRO BIOPHARMA, INC.
111 Oyster Point Boulevard
South San Francisco, California, 94080
NOTICE OF ANNUAL MEETING OF STOCKHOLDERS
To Be Held June 6, 2024
To Our Stockholders:
NOTICE IS HEREBY GIVEN that the 2024 Annual Meeting of Stockholders of Sutro Biopharma, Inc. will be held via a virtual meeting. You will be able to participate in the 2024 Annual Meeting and vote during the 2024 Annual Meeting via live webcast by visiting www.virtualshareholdermeeting.com/STRO2024 on Thursday, June 6, 2024 at 9:00 AM Pacific Time. We believe that a virtual stockholder meeting provides greater access to those who may want to attend, and therefore we have chosen this over an in-person meeting. It is important that you retain a copy of the control number found on the proxy card or voting instruction form, or included in the e-mail to you if you received the proxy materials by e-mail, as such number will be required in order for stockholders to gain access to the virtual meeting.
We are holding the meeting for the following purposes, which are more fully described in the accompanying proxy statement:
In addition, stockholders may be asked to consider and vote upon such other business as may properly come before the meeting or any adjournment or postponement thereof.
Only stockholders of record at the close of business on April 10, 2024 are entitled to receive notice of, and to vote at, the meeting and any adjournments thereof. This Notice of Internet Availability of Proxy Materials (“Notice of Internet Availability”) and the accompanying proxy statement are being mailed out to stockholders as of the record date beginning on or about April 26, 2024.
For ten days prior to the meeting, a complete list of the stockholders entitled to vote at the meeting will be available upon request by any stockholder for any purpose relating to the meeting. Stockholders can request the list of stockholders through our investor relations website at https://ir.sutrobio.com/contact-ir .
Your vote as a Sutro Biopharma, Inc. stockholder is very important. Each share of common stock that you own represents one vote.
For questions regarding your stock ownership, you may contact our Investor Relations group at IR@sutrobio.com or, if you are a registered holder, our transfer agent, Equiniti Trust Company, LLC by email through their website at https://www.equiniti.com/us or by phone at (800) 937-5449. Whether or not you expect to attend the meeting, we encourage you to read the proxy statement and vote through the internet or by telephone, or to request, sign and return your proxy card as soon as possible, so that your shares may be represented at the meeting. For specific instructions on how to vote your shares, please refer to the section entitled “General Proxy Information” in the proxy statement.
|
|
By Order of the Board of Directors, |
|
|
William J. Newell |
Chief Executive Officer |
South San Francisco, California
April 26, 2024
Important Notice Regarding the Availability of Proxy Materials for the virtual Annual Meeting of Stockholders to be held on June 6, 2024: the Proxy Statement and our 2023 Annual Report on Form 10-K are available at http://ir.sutrobio.com. You will need the control number included on your proxy card or voting instruction form, or included in the e-mail to you if you received the proxy materials by e-mail, as such number will be required in order for stockholders to gain access to the virtual meeting.
SUTRO BIOPHARMA, INC.
PROXY STATEMENT FOR 2024 ANNUAL MEETING OF STOCKHOLDERS
TABLE OF CONTENTS
|
|
|
|
|
|
|
Page |
|
|
|
1 |
|
|
|
|
|
|||
|
1 |
|
|
|
|
|
|||
|
1 |
|
|
|
|
|
|||
|
2 |
|
|
|
|
|
|||
|
5 |
|
|
|
|
|
|||
|
11 |
|
|
|
|
|
|||
PROPOSAL NO. 2 RATIFICATION OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM |
|
17 |
|
|
|
|
|||
|
18 |
|
|
|
|
|
|||
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT |
|
19 |
|
|
|
|
|||
|
22 |
|
|
|
|
|
|||
|
23 |
|
|
|
|
|
|||
|
38 |
|
|
|
|
|
|
|
|
|
40 |
|
|
|
|
|
|||
PROPOSAL NO. 3 NON-BINDING ADVISORY VOTE ON THE COMPENSATION OF OUR NAMED EXECUTIVE OFFICERS |
|
41 |
|
|
|
||||
PROPOSAL NO. 4 AMENDMENT TO OUR 2018 EMPLOYEE STOCK PURCHASE PLAN |
|
42 |
|
|
|
|
|
|
|
|
46 |
|
|
|
|
|
|||
|
47 |
|
|
|
|
|
|||
|
48 |
|
|
|
|
|
|
|
|
|
49 |
|
|
|
SUTRO BIOPHARMA, INC.
111 Oyster Point Boulevard
South San Francisco, California, 94080
PROXY STATEMENT FOR THE 2024 ANNUAL MEETING OF STOCKHOLDERS
April 26, 2024
INFORMATION ABOUT SOLICITATION AND VOTING
The accompanying proxy is solicited on behalf of the Board of Directors of Sutro Biopharma, Inc. (Sutro Biopharma or the Company) for use at Sutro Biopharma’s 2024 Annual Meeting of Stockholders (Annual Meeting) to be held via a virtual meeting. You will be able to participate in the Annual Meeting and vote during the Annual Meeting via live webcast by visiting www.virtualshareholdermeeting.com/STRO2024 on Thursday, June 6, 2024 at 9:00 AM Pacific Time and any adjournment or postponement thereof. We believe that a virtual stockholder meeting provides greater access to those who may want to attend, and therefore we have chosen this format over an in-person meeting. This approach also lowers costs and aligns with our broader sustainability goals. You will need the control number included on your proxy card or voting instruction form, or included in the e-mail to you if you received the proxy materials by e-mail, as such number will be required in order for stockholders to gain access to the virtual meeting. We are making this proxy statement, the accompanying form of proxy and our Annual Report on Form 10‑K for the year ended December 31, 2023 first available to stockholders on or about April 26, 2024. An electronic copy of this proxy statement and Annual Report on Form 10‑K are available at http://ir.sutrobio.com.
INTERNET AVAILABILITY OF PROXY MATERIALS
Under rules adopted by the Securities and Exchange Commission (the “SEC”), we are furnishing proxy materials to our stockholders primarily via the Internet, instead of mailing printed copies to each stockholder. On or about April 26, 2024, we expect to send to our stockholders a Notice of Internet Availability containing instructions on how to access our proxy materials, including our proxy statement and our Annual Report on Form 10-K. The Notice of Internet Availability also provides instructions on how to vote and includes instructions on how to receive paper copies of the proxy materials by mail, or an electronic copy of the proxy materials by email.
This process is designed to reduce our environmental impact and lower the costs of printing and distributing our proxy materials while providing our stockholders timely access to this important information. If you would prefer to receive printed proxy materials, please follow the instructions included in the Notice of Internet Availability.
GENERAL INFORMATION ABOUT THE MEETING
Purpose of the Meeting
At the meeting, stockholders will act upon the proposals described in this proxy statement. In addition, we will consider any other matters that are properly presented for a vote at the meeting. We are not aware of any other matters to be submitted for consideration at the meeting. If any other matters are properly presented for a vote at the meeting, the persons named in the proxy, who are officers of the company, have the authority in their discretion to vote the shares represented by the proxy.
Record Date; Quorum
Only holders of record of common stock at the close of business on April 10, 2024, the record date, will be entitled to vote at the meeting. At the close of business on April 10, 2024, 81,764,683 shares of common stock were outstanding and entitled to vote.
The holders of a majority of the voting power of the shares of stock entitled to vote at the meeting as of the record date must be present or represented by proxy at the meeting in order to hold the meeting and conduct business. This presence is called a quorum. Your shares are counted as present at the meeting if you are present and vote online at the virtual meeting or if you have properly submitted a proxy.
1
GENERAL PROXY INFORMATION
Voting Rights; Required Vote
Each holder of shares of common stock is entitled to one vote for each share of common stock held as of the close of business on April 10, 2024, the record date. You may vote all shares owned by you at such date, including (1) shares held directly in your name as the stockholder of record and (2) shares held for you as the beneficial owner in street name through a broker, bank, trustee or other nominee. Dissenters’ rights are not applicable to any of the matters being voted on.
Stockholder of Record: Shares Registered in Your Name. If on April 10, 2024, your shares were registered directly in your name with our transfer agent, Equiniti Trust Company LLC, then you are considered the stockholder of record with respect to those shares. As a stockholder of record, you may vote at the meeting, or vote in advance through the internet or by telephone, or if you request to receive paper proxy materials by mail, by filling out and returning the proxy card.
Beneficial Owner: Shares Registered in the Name of a Broker or Nominee. If on April 10, 2024, your shares were held in an account with a brokerage firm, bank or other nominee, then you are the beneficial owner of the shares held in street name. As a beneficial owner, you have the right to direct your broker on how to vote the shares held in your account, and your broker has enclosed or provided voting instructions for you to use in directing it on how to vote your shares. Because the brokerage firm, bank or other nominee that holds your shares is the stockholder of record, if you wish to attend the meeting and vote your shares, you must obtain a valid proxy from the firm that holds your shares giving you the right to vote the shares at the meeting.
Each director will be elected by a plurality of the votes cast by the holders of the shares present in person or represented by proxy at the meeting and entitled to vote on the election of directors. This means that the two individuals nominated for election to the Board of Directors at the meeting receiving the highest number of “FOR” votes will be elected. You may either vote “FOR” one, two or all of the nominees or “WITHHOLD” your vote with respect to one, two or all of the nominees. You may not cumulate votes in the election of directors. Approval of the ratification of the appointment of our independent registered public accounting firm requires the affirmative vote of the holders of a majority of the voting power of the shares of stock entitled to vote on such matter that are present in person or represented by proxy at the meeting and are voted for or against the matter. Approval, on a non-binding advisory basis, of the compensation of our named executive officers requires the affirmative vote of the holders of a majority of the voting power of the shares of stock entitled to vote on such matter that are present in person or represented by proxy at the meeting and are voted for or against the matter. Approval of an amendment to our 2018 ESPP (as amended and restated, the “Amended ESPP”) to (i) increase the overall limit on the number of shares that may be issued under the ESPP throughout its ten-year term, and (ii) make certain non-substantive clarifying revisions requires the affirmative vote of the holders of a majority of the voting power of the shares of stock entitled to vote on such matter that are present in person or represented by proxy at the meeting and are voted for or against the matter.
A proxy submitted by a stockholder may indicate that the shares represented by the proxy are not being voted (stockholder withholding) with respect to a particular matter or the stockholder may abstain from voting on the matter (abstention). In addition, a broker may not be permitted to vote on shares held in street name on a particular matter in the absence of instructions from the beneficial owner of the stock (broker non-vote). The shares subject to a proxy which are not being voted on a particular matter because of either stockholder withholding or broker non-votes will count for purposes of determining the presence of a quorum, but are not treated as votes cast and, therefore, will have no effect on the outcome of any of the proposals. Additionally, abstentions will count for purposes of determining the presence of a quorum, but are voted neither “for” nor “against” a matter, and, therefore, will have no effect on the outcome of any of the proposals.
Recommendations of the Board of Directors on Each of the Proposals Scheduled to be Voted on at the Meeting
The Board of Directors recommends that you vote FOR the election of each of the Class III directors named in this proxy statement (Proposal 1); FOR the ratification of the appointment of Ernst & Young LLP as our independent registered public accounting firm for the fiscal year ending December 31, 2024 (Proposal 2); FOR the approval, on a non-binding advisory basis, of the compensation of our named executive officers, as disclosed in this Proxy Statement (Proposal 3); and FOR the approval of the Amended ESPP to (i) revise the overall limit on the number of shares that may be issued under the ESPP throughout its ten-year term, and (ii) make certain non-substantive clarifying revisions (Proposal 4). .
None of the directors or executive officers has any substantial interest in any matter to be acted upon, other than elections to office with respect to the directors nominated in Proposal 1 and other than as referenced in Proposal 4-Approval of Amendment to 2018 Employee Stock Purchase Plan-Certain Interests of Employees.
2
Voting Instructions; Voting of Proxies
If you are a stockholder of record, you may:
Votes submitted through the internet or by telephone must be received by 11:59 p.m., Eastern Time, on June 5, 2024. Submitting your proxy, whether by telephone, through the internet or by mail if you requested or received a paper proxy card, will not affect your right to vote online should you decide to attend the virtual meeting. If you are not the stockholder of record, please refer to the voting instructions provided by your nominee to direct how to vote your shares. For Proposal 1, you may either vote “FOR” all of the nominees to the Board of Directors, or you may withhold your vote from any nominee you specify. For Proposal 2, you may vote “FOR” or “AGAINST” or “ABSTAIN” from voting. For Proposal 3, you may vote “FOR” or “AGAINST” or “ABSTAIN” from voting. For Proposal 4, you may vote “FOR” or “AGAINST” or “ABSTAIN” from voting. Your vote is important. Whether or not you plan to attend the meeting, we urge you to vote by proxy to ensure that your vote is counted.
All proxies will be voted in accordance with the instructions specified on the proxy card. If you sign a physical proxy card and return it without instructions as to how your shares should be voted on a particular proposal at the meeting, your shares will be voted in accordance with the recommendations of our Board of Directors stated above.
If your shares are held in an account with a brokerage firm, bank or other nominee, then you are deemed to be the beneficial owner of your shares and the broker that actually holds the shares for you is the record holder and is required to vote the shares it holds on your behalf according to your instructions. The proxy materials, as well as voting and revocation instructions, should have been forwarded to you by the bank, broker or other nominee that holds your shares. In order to vote your shares, you will need to follow the instructions that your bank, broker or other nominee provides you. The voting deadlines and availability of telephone and Internet voting for beneficial owners of shares held in street name will depend on the voting processes of the bank, broker or other nominee that holds your shares. Therefore, we urge you to carefully review and follow the voting instruction card and any other materials that you receive from that organization.
If you received a Notice of Internet Availability, please follow the instructions included on the notice on how to access and vote your proxy card. If you do not vote and you hold your shares in street name, and your broker does not have discretionary power to vote your shares, your shares may constitute “broker non-votes” (as described above) and will not be counted in determining the number of shares necessary for approval of the proposals. However, shares that constitute broker non-votes will be counted for the purpose of establishing a quorum for the meeting.
If you receive more than one proxy card or Notice of Internet Availability, your shares are registered in more than one name or are registered in different accounts. To make certain all of your shares are voted, please follow the instructions included on the Notice of Internet Availability on how to access and vote each proxy card. If you requested or received paper proxy materials by mail, please complete, sign, date and return each proxy card to ensure that all of your shares are voted.
Expenses of Soliciting Proxies
We will pay the expenses associated with soliciting proxies. Following the original distribution and mailing of the solicitation materials, we or our agents may solicit proxies by mail, email, telephone, facsimile, by other similar means, or in person. Our directors, officers and other employees, without additional compensation, may solicit proxies personally or in writing, by telephone, email or otherwise. Following the original distribution and mailing of the solicitation materials, we will request brokers, custodians, nominees and other record holders to forward copies of those materials to persons for whom they hold shares and to request authority for the exercise of proxies. In such cases, we, upon the request of the record holders, will reimburse such holders for their reasonable expenses. If you choose to access the proxy materials and/or vote through the internet, you are responsible for any internet access charges you may incur.
3
Revocability of Proxies
A stockholder of record who has given a proxy may revoke it at any time before the closing of the polls by the inspector of elections at the meeting by:
Please note, however, that if your shares are held of record by a brokerage firm, bank or other nominee, and you wish to revoke a proxy, you must contact that firm to revoke or change any prior voting instructions.
Electronic Access to the Proxy Materials
The Notice of Internet Availability will provide you with instructions regarding how to:
Choosing to receive your future proxy materials by email will reduce the impact of our annual meetings of stockholders on the environment and lower the costs of printing and distributing our proxy materials. If you choose to receive future proxy materials by email, you will receive an email next year with instructions containing a link to those materials and a link to the proxy voting site. Your election to receive proxy materials by email will remain in effect until you terminate it.
Voting Results
Voting results will be tabulated and certified by the inspector of elections appointed for the meeting. The final results will be tallied by the inspector of elections and filed with the SEC in a Current Report on Form 8-K within four business days of the meeting.
4
CORPORATE GOVERNANCE STANDARDS AND DIRECTOR INDEPENDENCE
We are committed to good corporate governance practices. These practices provide an important framework within which our Board of Directors and management pursue our strategic objectives for the benefit of our stockholders.
Corporate Governance Guidelines
Our Board of Directors has adopted Corporate Governance Guidelines that set forth expectations for directors, director independence standards, Board Committee structure and functions, and other policies for the governance of the company. Our Corporate Governance Guidelines are available without charge on the investor relations section of our website at https://ir.sutrobio.com/corporate-governance/governance-overview.
Board Composition and Leadership Structure
Our Nominating and Corporate Governance Committee periodically considers the leadership structure of our Board of Directors and makes such recommendations to our Board of Directors as our Nominating and Corporate Governance Committee deems appropriate. The positions of Chief Executive Officer and Chair of our Board of Directors are currently held by two different individuals (William J. Newell and Connie Matsui, respectively). This structure allows our Chief Executive Officer to focus on our day-to-day business while our Chair leads our Board of Directors in its fundamental role of providing advice to, and independent oversight of, management. Mr. Newell’s significant managerial experience working with and serving in various executive positions in the life sciences companies makes him well-suited for this day-to-day operational role, while Ms. Matsui’s extensive experience advising life sciences companies with respect to corporate strategy, product development, sales and marketing and operations allows her to perform an oversight function separate from management. Our Board of Directors believes such separation is appropriate, as it enhances the accountability of the Chief Executive Officer to the Board of Directors and strengthens the independence of the Board of Directors from management. Any changes to the leadership structure of our Board of Directors, if made, will be promptly disclosed on the investor relations section of our website and in our proxy materials. Our Board of Directors, in its sole discretion, may seek input from our stockholders on the leadership structure of the Board of Directors.
Board’s Role in Risk Oversight
Our Board of Directors believes that open communication between management and the Board of Directors is essential for effective risk management and oversight. Our Board of Directors meets with our Chief Executive Officer and other members of the senior management team at regular quarterly Board of Director meetings, and at ad hoc meetings when deemed appropriate, where, among other topics, they discuss strategy and risks in the context of reports from the management team and evaluate the risks inherent in significant transactions. While our Board of Directors is ultimately responsible for risk oversight, our Board Committees assist the Board of Directors in fulfilling its oversight responsibilities in certain areas of risk. The Audit Committee assists our Board of Directors in fulfilling its oversight responsibilities with respect to risk management in the areas of internal controls over financial reporting, disclosure controls and procedures, and information security. The Compensation Committee assists our Board of Directors in assessing risks created by the incentives inherent in our compensation policies. The Nominating and Governance Committee assists our Board of Directors in fulfilling its oversight responsibilities with respect to the management of corporate, legal and regulatory risk and environmental, social and governance, or ESG, concerns. The Science and Technology Committee assists our Board of Directors in fulfilling its oversight responsibilities with respect to our research and development and platform programs.
Cybersecurity Risk Oversight
Our Board of Directors recognizes the critical importance of maintaining the trust and confidence of our patients, business partners and employees. Our Board of Directors is actively involved in oversight of our risk management program, and cybersecurity represents an important component of our overall approach to enterprise risk management (“ERM”). Through our ERM program, risks are identified, assessed and managed at the organization level, mission and business process level, and information system level. Our cybersecurity program, policies and procedures are fully integrated into our ERM program and are maintained in accordance with industry good standards. Our Board of Directors’ oversight of cybersecurity risk management is supported by our Audit Committee, which regularly interacts with our executive leadership, including our Chief Executive Officer, Chief Financial Officer and our General Counsel and other key officers.
We have implemented a comprehensive, cross-functional approach to identifying, preventing and mitigating cybersecurity threats and incidents, while also implementing controls and procedures that provide for the prompt escalation of certain cybersecurity incidents so that decisions regarding the public disclosure and reporting of such incidents can be made by management in a timely manner. We also maintain a comprehensive, risk-based approach to identifying and overseeing cybersecurity risks presented by third parties,
5
including vendors, service providers and other external users of our systems, as well as the systems of third parties that could adversely impact our business in the event of a cybersecurity incident affecting those third-party systems. Further information relating to cybersecurity and information security is contained in the section titled “Cybersecurity” in our Annual Report on Form 10-K for the year ended December 31, 2023.
Director Independence
Our common stock is listed on the Nasdaq Global Market. Under the rules of the Nasdaq Stock Market, independent directors must constitute a majority of a listed company’s Board of Directors. In addition, the rules of the Nasdaq Stock Market require that, subject to specified exceptions, each member of a listed company’s Audit, Compensation and Nominating and Governance Committees must be an “independent director”. Under the rules of the Nasdaq Stock Market, a director will only qualify as an “independent director” if, in the opinion of that company’s Board of Directors, that person does not have a relationship that would interfere with the exercise of independent judgment in carrying out the responsibilities of a director. Additionally, Compensation Committee members must not have a relationship with the listed company that is material to the director’s ability to be independent from management in connection with the duties of a Compensation Committee member.
Audit Committee members must also satisfy the independence criteria set forth in Rule 10A-3 under the Securities Exchange Act of 1934, as amended (Exchange Act). In order to be considered independent for purposes of Rule 10A-3, a member of an Audit Committee of a listed company may not, other than in his or her capacity as a member of the Audit Committee, the Board of Directors or any other Board Committee: (i) accept, directly or indirectly, any consulting, advisory or other compensatory fee from the listed company or any of its subsidiaries or (ii) be an affiliated person of the listed company or any of its subsidiaries.
Our Board of Directors has undertaken a review of the independence of each director and considered whether each director has a material relationship with us that could compromise his or her ability to exercise independent judgment in carrying out his or her responsibilities. As a result of this review, our Board of Directors determined that Connie Matsui, Michael Dybbs, John Freund, Heidi Hunter, Joseph Lobacki, James Panek, Daniel Petree, and Jon Wigginton representing eight of our nine incumbent directors, are “independent directors” as defined under the applicable rules and regulations of the SEC and the listing requirements and rules of the Nasdaq Stock Market. In making these determinations, our Board of Directors reviewed and discussed information provided by the directors and us with regard to each directors’ business and personal activities and relationships as they may relate to us and our management, including the beneficial ownership of our capital stock by each non-employee director and any affiliates.
Board of Directors and Committee Self-Evaluations
Our Board of Directors is committed to a robust self-evaluation process designed for continuous improvement. To achieve this, our Board of Directors conducts an annual self-evaluation for itself and its committees. As part of this process, each member of the Board of Directors completes a survey to provide feedback on the processes, structure, composition and effectiveness of the Board of the Directors. The survey is a detailed written questionnaire designed to help the Board of Directors assess the performance of the Board of Directors and its committees, their own individual performance and the individual performances of fellow directors. The feedback received is shared first with the Nominating and Corporate Governance Committee, and then made available to the individual directors and the full Board of Directors.
Committees of Our Board of Directors
Our Board of Directors has established an Audit Committee, a Compensation Committee, a Nominating and Governance Committee and a Science and Technology Committee, each of which has the composition and responsibilities described below. Members serve on these Committees until their resignation or until otherwise determined by our Board of Directors. Each of these Committees has a written charter, copies of which are available without charge on the investor relations section of our website at https://ir.sutrobio.com/corporate-governance/governance-overview under the heading “Governance.”
Audit Committee
Our Audit Committee is composed of Ms. Hunter, Dr. Freund, and Mr. Lobacki. Ms. Hunter is the Chair of our Audit Committee. The composition of our Audit Committee meets the requirements for independence under the current Nasdaq Stock Market and SEC rules and regulations. Each member of our Audit Committee is financially literate. In addition, our Board of Directors has determined that Ms. Hunter is an “Audit Committee financial expert” as defined in Item 407(d)(5)(ii) of Regulation S-K promulgated under the Securities Act. Our Audit Committee is directly responsible for, among other things:
6
Compensation Committee
Our Compensation Committee is composed of Mr. Lobacki, Mr. Petree, and Dr. Wigginton. Mr. Lobacki is the Chair of our Compensation Committee. The composition of our Compensation Committee meets the requirements for independence under the current Nasdaq Stock Market and SEC rules and regulations. Our Compensation Committee is responsible for, among other things:
The Compensation Committee has the sole authority and responsibility, subject to any approval by the Board of Directors which the Compensation Committee or legal counsel determines to be desirable or required by applicable law or the Nasdaq rules, to determine all aspects of executive compensation packages for the Chief Executive Officer and other executive officers. The Compensation Committee also makes recommendations to our Board of Directors regarding the form and amount of compensation of non-employee directors. The Compensation Committee may take into account the recommendations of the Chief Executive Officer with respect to compensation of the other executive officers, and the recommendations of the Board of Directors or any member of the Board of Directors with respect to compensation of the Chief Executive Officer and other executive officers.
The Compensation Committee engaged an independent executive compensation consulting firm, Frederic W. Cook & Co., Inc., or FW Cook, to evaluate our executive compensation and Board of Directors compensation program and practices and to provide advice and ongoing assistance on these matters for the fiscal year ended December 31, 2023. Specifically, FW Cook was engaged to:
Representatives of FW Cook met informally with the Chair of the Compensation Committee and attended the regular meetings of the Compensation Committee, including executive sessions from time to time without any members of management present. During the fiscal year ended December 31, 2023, FW Cook worked with the Compensation Committee to assist the Committee in satisfying its responsibilities and undertook no projects for management without the Committee’s prior approval. The Compensation Committee has determined that none of the work performed by FW Cook during the fiscal year ended December 31, 2023 raised any conflict of interest.
Nominating and Governance Committee
Our Nominating and Governance Committee is composed of Ms. Matsui, Mr. Panek and Mr. Petree. Ms. Matsui is the Chair of our Nominating and Governance Committee. Our Nominating and Governance Committee is responsible for, among other things:
7
Science and Technology Committee
Our Science and Technology Committee is composed of Dr. Wigginton, Dr. Dybbs, Ms. Hunter and Mr. Panek. Dr. Wigginton is the Chair of our Science and Technology Committee. Our Science and Technology Committee is responsible for, among other things:
Code of Business Conduct and Ethics
Our Board of Directors has adopted a code of business conduct and ethics that applies to all of our employees, officers and directors, including our Chief Executive Officer, Chief Financial Officer and other executive and senior financial officers. We intend to disclose future amendments to certain provisions of our code of business conduct, or waivers of these provisions, on our website or in public filings. The full text of our code of business conduct is posted on the investor relations section of our website at https://ir.sutrobio.com/corporate-governance/governance-overview.
Corporate Social Responsibility
We believe that corporate social responsibility (“CSR”) initiatives are important to our business and to creating sustainable value for our stockholders and wider stakeholder group. Our Board of Directors and management are committed to these initiatives and believe these efforts will benefit our employees, partners, and the communities in which we operate.
Anti-hedging
We have adopted an Insider Trading Policy that applies to all of our employees, officers and directors, including our Chief Executive Officer and other executive officers, which prohibits such individuals from purchasing financial instruments, or otherwise engaging in transactions, that hedge or offset, or are designed to hedge or offset, any decrease in market value of our common stock, such as prepaid variable forward contracts, equity swaps, collars, forward sale contracts and exchange funds.
Compensation Committee Interlocks and Insider Participation
During 2023, Mr. Lobacki, Mr. Petree, and Dr. Wigginton served on our Compensation Committee. None of our current executive officers has served as a member of the Board of Directors, or as a member of the Compensation or similar Committee, of any entity that has one or more executive officers who served on our Board of Directors or Compensation Committee during the fiscal year ended December 31, 2023.
Board and Committee Meetings and Attendance
The Board of Directors and its Committees meet regularly throughout the year and also hold special meetings and act by written consent from time to time. During 2023, the Board of Directors held seven meetings including video conference meetings; the Audit Committee held four meetings; the Compensation Committee held six meetings; the Science and Technology Committee held three meetings; and the Nominating and Governance Committee held four meetings. During 2023, none of the directors attended fewer than 75% of the aggregate of the total number of meetings held by the Board of Directors during his or her tenure and the total number of meetings held by all Committees of the Board of Directors on which such director served during his or her tenure. The independent members of the Board of Directors also meet separately without management directors on a regular basis to discuss such matters as the independent directors consider appropriate.
8
Board Attendance at Annual Stockholders’ Meeting
We invite and encourage each member of our Board of Directors to attend our annual meetings of stockholders. All of our directors attended our 2023 annual meeting of stockholders, which was held virtually. We do not have a formal policy requiring attendance of our annual meetings of stockholders by the members of our Board of Directors.
Communication with Directors
Stockholders and interested parties who wish to communicate with our Board of Directors, non-management members of our Board of Directors as a group, a Committee of the Board of Directors or a specific member of our Board of Directors (including our Chair) may do so by letters addressed to:
Sutro Biopharma, Inc.
c/o Corporate Secretary
111 Oyster Point Boulevard
South San Francisco, California, 94080
All communications by letter addressed to the attention of our Corporate Secretary will be reviewed by the Corporate Secretary and provided to the members of the Board of Directors unless such communications are unsolicited items, sales materials and other routine items and items unrelated to the duties and responsibilities of the Board of Directors.
Considerations in Evaluating Director Nominees
The Nominating and Governance Committee is responsible for identifying, considering and recommending candidates to the Board of Directors for Board membership. A variety of methods are used to identify and evaluate director nominees, with the goal of maintaining and further developing a diverse, experienced and highly qualified Board of Directors. Candidates may come to our attention through current members of our Board of Directors, professional search firms, stockholders or other persons.
The Nominating and Governance Committee will recommend to the Board of Directors for selection all nominees to be proposed by the Board of Directors for election by the stockholders, including approval or recommendation of a slate of director nominees to be proposed by the Board of Directors for election at each annual meeting of stockholders, and will recommend all director nominees to be appointed by the Board of Directors to fill interim director vacancies.
Our Board of Directors encourages selection of directors who will contribute to the company’s overall corporate goals. The Nominating and Governance Committee may from time to time review and recommend to the Board of Directors the desired qualifications, expertise and characteristics of director candidates, including such factors as business experience, diversity and personal skills in life sciences and biotechnology, finance, marketing, financial reporting and other areas that are expected to contribute to an effective Board of Directors. Exceptional candidates who do not meet all of these criteria may still be considered. In evaluating potential candidates for the Board of Directors, the Nominating and Governance Committee considers these factors in the light of the specific needs of the Board of Directors at that time.
In addition, under our Corporate Governance Guidelines, a director is expected to spend the time and effort necessary to properly discharge such director’s responsibilities. Accordingly, a director is expected to regularly attend meetings of the Board of Directors and Committees on which such director sits, and to review prior to meetings material distributed in advance for such meetings. Thus, the number of other public company boards and other boards (or comparable governing bodies) on which a prospective nominee is a member, as well as his or her other professional responsibilities, will be considered. Also, under our Corporate Governance Guidelines, there are no limits on the number of three-year terms that may be served by a director. However, in connection with evaluating recommendations for nomination for reelection, the Nominating and Governance Committee considers director tenure. We value diversity on a company-wide basis but have not yet adopted a specific policy regarding Board diversity, though we plan to comply with any applicable legal and listing requirements for director diversity.
Stockholder Recommendations for Nominations to the Board of Directors
The Nominating and Governance Committee will consider properly submitted stockholder recommendations for candidates for our Board of Directors who meet the minimum qualifications as described above. The Nominating and Governance Committee does not intend to alter the manner in which it evaluates candidates, including the minimum criteria set forth above, based on whether or not the candidate was recommended by a stockholder. A stockholder of record can nominate a candidate for election to the Board of Directors by complying with the procedures in Article I, Section 1.12 of our Amended and Restated Bylaws. Any eligible stockholder who wishes to submit a nomination should review the requirements in the Amended and Restated Bylaws on nominations by stockholders.
9
Any nomination should be sent in writing to our Corporate Secretary, Sutro Biopharma, Inc., 111 Oyster Point Boulevard, South San Francisco, California, 94080. Submissions must include the full name of the proposed nominee, complete biographical information, a description of the proposed nominee’s qualifications as a director, other information regarding the nominee and proposing stockholder as specified in our Amended and Restated Bylaws, and certain representations regarding the nomination. Any such submission must be accompanied by the written consent of the proposed nominee to be named as a nominee and to serve as a director if elected. These candidates are evaluated at meetings of the Nominating and Governance Committee and may be considered at any point during the year. If any materials are provided by a stockholder in connection with the recommendation of a director candidate, such materials are forwarded to the Nominating and Governance Committee.
Additional information regarding the process for properly submitting stockholder nominations for candidates for membership on our Board of Directors is set forth below under “Stockholder Proposals to Be Presented at Next Annual Meeting.” In addition to satisfying the foregoing requirements under our Amended and Restated Bylaws, to comply with the universal proxy rules, stockholders who intend to solicit proxies in support of director nominees other than the Company’s nominees for the 2025 Annual Meeting of Stockholders must provide notice that sets forth the information required by Rule 14a-19 under the Exchange Act.
10
PROPOSAL NO. 1
ELECTION OF CLASS III DIRECTORS
Our Board of Directors is divided into three classes. Each class serves for three years, with the terms of office of the respective classes expiring in successive years. Directors and director nominees in Class III will stand for election at this meeting. The terms of office of directors in Class I and Class II do not expire until the annual meetings of stockholders to be held in 2025 and 2026, respectively. Our Nominating and Governance Committee recommended to our Board of Directors, and our Board of Directors nominated Mr. Lobacki and Mr. Petree, each an incumbent Class III director, for election as Class III directors at the Annual Meeting. At the recommendation of our Nominating and Governance Committee, our Board of Directors proposes that each of the Class III nominees be elected as a Class III director for a three-year term expiring at the annual meeting of stockholders to be held in 2027 and until such director’s successor is duly elected and qualified or until such director’s earlier resignation or removal.
Each director will be elected by a plurality by the holders of the shares present in person or represented by proxy at the meeting and entitled to vote on the election of directors. This means that the two individuals nominated for election to the Board of Directors at the Annual Meeting receiving the highest number of “FOR” votes will be elected. You may either vote “FOR” one, two, or all of the nominees or “WITHHOLD” your vote with respect to one, two, or all of the nominees. A “WITHHOLD” vote will have the same effect as an abstention. Shares represented by proxies will be voted “FOR” the election of each of the Class III nominees, unless the proxy is marked to withhold authority to so vote. You may not cumulate votes in the election of directors. If any nominee for any reason is unable to serve, the proxies may be voted for such substitute nominee as the proxy holders, who are officers of our company, might determine. Each nominee has consented to being named in this proxy statement and to serve if elected. Proxies may not be voted for more than two directors.
Nominees to the Board of Directors
The nominees and their ages as of March 31, 2024 are provided in the table below. Additional biographical information for each nominee is set forth in the text below the table.
Name |
|
Age |
|
Class |
Joseph M. Lobacki (1)(2) |
|
65 |
|
Class III Director |
Daniel H. Petree (2)(3) |
|
68 |
|
Class III Director |
(1) Member of our Audit Committee
(2) Member of our Compensation Committee
(3) Member of our Nominating and Governance Committee
Joseph M. Lobacki has served as a member of our Board of Directors since February 2017. From January 2020 to January 2024, Mr. Lobacki served as president and CEO of Artax, a private biopharmaceutical company developing treatments for autoimmune and inflammatory diseases focused on modulating the T-Cell Receptor response to antigen stimulation. Since January 2024, Mr. Lobacki has served in an advisory role at Artax, and as an independent consultant to Biotech companies. Previously, Mr. Lobacki served as Executive Vice President and Chief Commercial Officer for Verastem, Inc., a biopharmaceutical company focused on the development and commercialization of therapies for the treatment of hematologic malignancies. From November 2016 to December 2017, Mr. Lobacki served as Chief Operating Officer for Crestovo, a clinical-stage biopharmaceutical company focused on microbiome therapies. From 2014 to 2016, Mr. Lobacki served as Chief Commercial Officer at Medivation, Inc., a biopharmaceutical company focused on development of novel therapies for the treatment of serious diseases. From 2012 to 2014, Mr. Lobacki also served as General Manager of Oncology at Idera Pharmaceuticals, Inc., a biopharmaceutical company focused on therapies for cancer and rare diseases. Previously, Mr. Lobacki served as Senior Vice-President and Chief Commercial Officer at Micromet, Inc., Senior Vice-President and General Manager of US Transplant and Oncology at Genzyme Corporation and in various other positions at SangStat Medical Corporation, Cell Pathways, Inc., Rhone-Poulenc Rorer and Lederle Laboratories. Mr. Lobacki previously served on the Board of Directors of Celator Pharmaceuticals Inc. Mr. Lobacki received a B.S. in Biology from Boston College and a B.S. in Pharmacy from the Massachusetts College of Pharmacy. We believe that Mr. Lobacki is qualified to serve on our Board of Directors because of his extensive biopharmaceutical managerial and commercial experience, including his positions of Chief Commercial Officer, Chief Operating Officer, Executive Vice President and Chief Executive Officer of biopharma companies. His broad understanding of medical affairs and commercial development due to his experiences contributes an important perspective to our Board of Directors’ discussions regarding commercial strategy, business development and human capital management matters.
Daniel H. Petree, has served as a member of our Board of Directors since August 2009. In April 2012, Mr. Petree co-founded Four Oaks Partners Consulting, LLC, which provided transaction advisory services to small and medium-sized life science companies until
11
2021 and in 2000, Mr. Petree co-founded P2 Partners, LLC, Four Oaks' predecessor in the same business. Before co-founding P2 Partners, Mr. Petree served as President and Chief Operating Officer of Axys Pharmaceuticals, Inc., Executive Vice President and Chief Financial Officer of Arris Pharmaceuticals, Incorporated and Vice President of Business Development at TSI Corporation and
was a corporate and securities lawyer. Mr. Petree previously served on the boards of directors of Lpath, Inc., Biocept, Inc. and Cypress Bioscience, Inc. along with a number of privately held biotechnology companies. Mr. Petree received an A.B. in History and Political Science from Stanford University and a J.D. from the University of Michigan Law School. We believe that Mr. Petree is qualified to serve on our Board of Directors because of his deep knowledge and expertise in structuring and negotiating pharmaceutical partnering arrangements and strategic transactions. His significant business development and financing expertise, and in particular his experience with asset monetization, provides our Board of Directors with valuable insight into financing and growth strategies for us.
Continuing Directors
The directors who are serving for terms that end following the Annual Meeting and their ages as of March 31, 2024 are provided in the table below. Additional biographical information for each nominee is set forth in the text below the table.
Name |
|
Age |
|
Class |
Michael Dybbs, Ph.D.(1) |
|
49 |
|
Class I Director |
John G. Freund, M.D. (2) |
|
70 |
|
Class I Director |
Heidi Hunter(1)(2) |
|
65 |
|
Class I Director |
Jon Wigginton, M.D.(1)(3) |
|
62 |
|
Class I Director |
Connie Matsui (4) |
|
70 |
|
Class II Director |
William J. Newell |
|
66 |
|
Class II Director |
James Panek (1)(4) |
|
71 |
|
Class II Director |
(1) Member of our Science and Technology Committee
(2) Member of our Audit Committee
(3) Member of our Compensation Committee
(4) Member of our Nominating and Governance Committee
Michael Dybbs, Ph.D., has served as a member of our Board of Directors since July 2018. Dr. Dybbs is currently a partner at Samsara BioCapital, where he has worked since March 2017. Prior to joining Samsara, Dr. Dybbs was a partner at New Leaf Venture Partners, where he worked from May 2009 until September 2016. Before joining New Leaf Venture Partners, Dr. Dybbs was a principal at the Boston Consulting Group. Dr. Dybbs currently serves on the boards of directors of Nkarta Therapeutics (NKTX) and several private companies. Dr. Dybbs previously served on the boards of directors of Versartis, Inc., Dimension Therapeutics, Inc., and multiple private companies. Dr. Dybbs received an A.B. in biochemical sciences from Harvard College and a Ph.D. in molecular biology from University of California, Berkeley, where he was awarded a Howard Hughes Medical Institute fellowship. We believe that Dr. Dybbs is qualified to serve on our Board of Directors because of his financial and strategic expertise as well as his deep understanding of the life sciences sector developed over years of investing and advising companies of all sizes. Our Board of Directors particularly values Dr. Dybbs’s experience in evaluation of and development of messaging strategies for clinical data.
John G. Freund, M.D., has served as a member of our Board of Directors since February 2014. Dr. Freund founded Skyline Ventures, a venture capital firm, in September 1997, where he served as a Managing Director beginning with its founding. Prior to founding Skyline, Dr. Freund served as Managing Director at Chancellor Capital Management, co-founded Intuitive Surgical, Inc., served in various positions at Acuson Corporation, most recently Executive Vice President, was a general partner at Morgan Stanley Venture Partners and co-founded the Healthcare Group in the Corporate Finance Department of Morgan Stanley. In 2016, Dr. Freund co-founded and was CEO of Arixa Pharmaceuticals, Inc. an antibiotic company, which was acquired by Pfizer in 2020. Dr. Freund currently serves on the boards of directors of Collegium Pharmaceutical, Inc., SI Bone, Inc. and fourteen U.S. registered investment funds managed by affiliates of Capital Group, Inc. Dr. Freund is a member of the Advisory Board for the Harvard Business School Healthcare Initiative. Dr. Freund previously served on the boards of directors of several publicly traded companies, including Proteon Therapeutics, Inc. and XenoPort, Inc., where he was Chairman, Concert Pharmaceuticals, Inc., Tetraphase Pharmaceuticals, Inc., MAP Pharmaceuticals, Inc. and MAKO Surgical Corp. Dr. Freund received an A.B. in History from Harvard College, an M.D. from Harvard Medical School and an M.B.A. from Harvard Business School, where he was a Baker Scholar. We believe that Dr. Freund is qualified to serve on our Board of Directors because of his extensive experience in operating and financing biopharmaceutical companies. In particular, Dr. Freund’s extensive experience leading companies focused in diverse areas from novel antibiotic discovery to medical robotic technology, together with his experience in developing and financing companies, provides our Board of Directors with valuable strategic and operational insights.
12
Heidi Hunter has served as a member of our Board of Directors since November 2021. Ms. Hunter is the former President of Cardinal Health Specialty Solutions, a portfolio of specialty healthcare businesses. Prior to Cardinal Health, Ms. Hunter served as Senior Vice President for UCB (Union Chimique Belge), a multinational biopharmaceutical company with a primary focus on neurology and immunology disorders from September 2015 to September 2020. Ms. Hunter also served as Senior Vice President and General Manager of Boehringer lngelheim, a pharmaceutical company, from 2011 to 2015. Prior to Boehringer lngelheim, Ms. Hunter held similar roles in sales and marketing at Ciba-Geigy (today part of Novartis), Centocor – a J&J company and Wyeth Pharmaceuticals LLC (today part of Pfizer) where she led their oncology business. Ms. Hunter also serves on the Board of Directors of Vicore Pharma Holding AB, Bavarian Nordic and IO Biotech. Ms. Hunter received a B.A in Economics from the University of Michigan and a M.B.A. from the University of Chicago - Booth School of Business. We believe that Ms. Hunter is qualified to serve on our Board of Directors because of her extensive experience in building and developing talent and high-performance teams in the pharmaceutical sector with the skills, motivation, and culture to achieve sustainable results. Ms. Hunter’s experience in various senior management positions, including as the former President of Cardinal Health Specialty Solutions, a portfolio of specialty healthcare businesses, strengthens our Board of Directors’ ability to oversee the important human capital management element of our company.
Jon Wigginton, M.D., has served as a member of our Board of Directors since November 2020. Dr. Wigginton currently serves as President, Research and Development at Bright Peak Therapeutics. Prior to that role, he was Senior Advisor and Chairman of the SAB at Cullinan Oncology, Inc., having served previously as the Chief Medical Officer focused on developing oncology therapeutics. From 2013 to 2020, Dr. Wigginton served as Senior Vice President, Clinical Development and Chief Medical Officer for MacroGenics, Inc., a clinical-stage biopharmaceutical company focused on discovering and developing innovative oncology therapeutics, including CD3-based bispecifics, monoclonal antibodies, and antibody drug conjugates.. From 2008 to 2013, Dr. Wigginton served as the Therapeutic Area Head, Immuno-Oncology, Early Clinical Research and Executive Director, Discovery Medicine-Clinical Oncology at Bristol-Myers. There, he led early clinical development of the BMS Immuno-Oncology portfolio, including checkpoint inhibitors such as anti-PD-1 (Nivolumab)/Opdivo®) and anti-PD-L1 (BMS-936559), checkpoint inhibitor-based combinations including anti-CTLA-4 (Yervoy®)/anti-PD-1 (Opdivo®) among others, and a spectrum of other immune-oncology agents. From 2006 to 2008, Dr. Wigginton served as the Director of Clinical Oncology at Merck Research Laboratories. During his academic career, Dr. Wigginton has held several positions at the National Cancer Institute Center for Cancer Research, including Head of Investigational Biologics Section, Pediatric Oncology Branch. Dr. Wigginton also served as a member of the Board of Directors of Checkmate Pharmaceuticals from January, 2022 through May, 2022, when the company was acquired by Regeneron. Dr. Wigginton previously served as President and as a member of the Board of directors of the Society for Immunotherapy of Cancer (non-profit). Dr. Wigginton received an M.D. and B.S. in Biology from the University of Michigan. We believe that Dr. Wigginton is qualified to serve on our Board of Directors because of his years of experience in clinical oncology and immunotherapy drug development. Dr. Wigginton’s significant experience in developing strategies that ultimately resulted in FDA approval of new treatment options offers a valuable perspective to our Board of Directors’ discussions regarding clinical development of new oncology therapeutics.
Connie Matsui has served as a member, and Chair, of our Board of Directors since June 2019 and brings over 18 years of general management experience in the biotechnology industry. From 2004 to 2009, Ms. Matsui served in various leadership positions at Biogen Idec, Inc., including as Executive Vice President, Knowledge and Innovation Networks and Executive Committee member. Prior to that, Ms. Matsui served in various leadership positions at IDEC Pharmaceuticals, a predecessor of Biogen Idec, including Senior Vice President; Collaboration Chair for the late-stage development and commercialization of rituximab (tradenames: Rituxan® and MabThera®) in partnership with Roche and Genentech; and Project Leader for Zevalin®, the first radioimmunotherapy approved by the U.S. FDA. Prior to entering the biotechnology industry, Ms. Matsui worked for Wells Fargo Bank in general management, marketing and human resources. Ms. Matsui currently serves on the boards of directors of Halozyme Therapeutics, Inc. and Artelo Biosciences, Inc., and has served on not-for-profit boards at the local, national and global level. Ms. Matsui received a B.A. and an M.B.A. from Stanford University. We believe that Ms. Matsui is qualified to serve on our Board of Directors because of her wealth of operational and managerial experience, including her extensive knowledge of the biotechnology industry, her service in other public company management teams and boards and her expertise in organizational and operational development. Her operational development experience includes serving as a Collaboration Chair for the late-stage development and commercialization of rituximab in the three-way IDEC Pharmaceuticals, Roche and Genentech collaboration. Her knowledge in these and other areas provide critical insights to our business, particularly as our Board of Directors considers our collaboration strategies through our proprietary XpressCF® and XpressCF+® platforms.
William J. Newell has served as our Chief Executive Officer and a member of our Board of Directors since January 2009. Previously, he served as the President of Aerovance, Inc., a biotechnology company focused on respiratory diseases, from 2006 to 2007. Mr. Newell has also served as the Chief Business Officer and Senior Vice President at QLT Inc., in several senior management positions at Axys Pharmaceuticals, Inc., and has experience as a corporate lawyer. Mr. Newell currently serves on the boards of directors of Biotechnology Innovation Organization’s (BIO) Health Section, Emerging Company Section and is a member of BIO’s executive committee. He was previously a member of California Life Science’s (CLS) executive committee and served on CLS’s board of directors. Mr. Newell received an A.B. in Government from Dartmouth College and a J.D. from the University of Michigan Law
13
School. We believe that Mr. Newell is qualified to serve on our Board of Directors because of his experience with various biotechnology companies, including working with and serving in various executive positions in life sciences companies.
James Panek has served as a member of our Board of Directors since January 2020. Since 2011, Mr. Panek has served as an Independent Consultant for various biopharmaceutical companies, and currently serves as Acting COO and Director for CHO Plus, a privately owned biotechnology company. From 2010 to 2011, Mr. Panek served as interim President, Chief Executive Officer and Principal Financial Officer at DiaDexus, Inc. From 2007 to 2010, Mr. Panek served as President, Chief Executive Officer and Principal Financial Officer for VaxGen, Inc., which subsequently became a subsidiary of DiaDexus, Inc. From 2002 to 2006, Mr. Panek served as Senior and Executive Vice President of VaxGen, Inc., and Co-Chief Executive Officer and Chairman of the Board for Celltrion Inc., then a VaxGen manufacturing joint venture in Inchon, Korea. In his role with Celltrion, Mr. Panek was responsible for the development and FDA licensure of the first large scale biopharmaceutical manufacturing facility in Asia. From 1982 to 2001, Mr. Panek served in various capacities with Genentech, Inc., including Senior Vice President, Product Operations, and Vice President, Manufacturing, Engineering and Facilities, where he led the development of the world’s largest biotechnology manufacturing facility and was responsible for all operations involved in supplying products for preclinical, clinical, and commercial use. Prior to joining Genentech, Mr. Panek spent six years with Eli Lilly in a variety of engineering and development positions. Mr. Panek currently serves on the board of directors of CHO Plus and previously served on the boards of directors of DiaDexus, Inc., VaxGen, Inc. and Celltrion Inc. Mr. Panek received a B.S. and an M.S. in Chemical Engineering from the University of Michigan. We believe that Mr. Panek is qualified to serve on our Board of Directors because of his 30 years of leadership experience in the biopharmaceutical industry. In particular, Mr. Panek has broad and deep extensive manufacturing and process development experience in our industry, including leading the construction, qualification and operation of Genentech’s pioneering large scale commercial manufacturing facility and his deep experience in managing supply chains and ensuring supply of preclinical, clinical and commercial products.
Family Relationships
There are no familial relationships among any of our directors and executive officers.
Board Diversity Matrix
Each of the Standing Committees of our Board of Directors has diverse representation. In addition, on our Board of Directors there are two directors who hold medical doctorates, one director who holds a doctorate in a scientific field, three directors who hold a Masters of Business Administration, and two directors who hold a juris doctorate. The table below provides certain highlights of the composition of our Board of Directors as disclosed by each current director. Each of the categories listed in the table below has the meaning set forth in Nasdaq Rule 5605(f). The diversity matrix of our Board of Directors for the year ended December 31, 2022 is available in our proxy statement for the 2023 annual meeting of our stockholders, filed with the SEC on April 28, 2023.
Board Diversity Matrix |
||||||||
Total Number of Directors |
|
|
|
|||||
|
|
Female |
|
Male |
|
Non-Binary |
|
Did Not Disclose Gender |
Part I: Gender Identity |
|
|||||||
Directors |
|
2 |
|
7 |
|
|
|
|
Part II: Demographic Background |
|
|||||||
Asian |
|
1 |
|
|
|
|
|
|
White |
|
1 |
|
7 |
|
|
|
|
Non-Employee Director Compensation
Our compensation arrangements for non-employee directors are reviewed periodically by our Compensation Committee and our Board of Directors. In addition, at the Compensation Committee’s direction, FW Cook, the Compensation Committee’s independent compensation consultant, provided a competitive analysis of director compensation levels, practices and design features as compared to the general market as well as to our compensation peer group. We last evaluated and adjusted compensation for our non-employee directors in 2023 and plan to do so again in 2024.
Our non-employee directors receive the following compensation pursuant to a program adopted by our Board of Directors:
14
Non-employee directors are also reimbursed for reasonable expenses incurred in serving as a director, including travel expenses for attending meetings of our Board of Directors.
The following table sets forth the compensation earned by or paid to the non-employee directors who served during 2023 for services provided during the year ended December 31, 2023. Mr. Newell, our Chief Executive Officer, received no compensation for his service as a director during 2023.
Name |
|
Fees Earned or Paid in Cash ($) |
|
|
Option Awards ($)(1) |
|
|
Total ($) |
|||
Connie Matsui |
|
$ |
85,000 |
|
|
$ |
88,105 |
|
|
$ |
173,105 |
Michael Dybbs, Ph.D. |
|
|
45,000 |
|
|
|
88,105 |
|
|
|
133,105 |
John Freund, M.D. |
|
|
50,000 |
|
|
|
88,105 |
|
|
|
138,105 |
Heidi Hunter |
|
|
62,170 |
|
|
|
88,105 |
|
|
|
150,275 |
Joseph Lobacki |
|
|
64,000 |
|
|
|
88,105 |
|
|
|
152,105 |
James Panek |
|
|
50,000 |
|
|
|
88,105 |
|
|
|
138,105 |
Daniel Petree |
|
|
52,000 |
|
|
|
88,105 |
|
|
|
140,105 |
Jon Wigginton, M.D. |
|
|
57,000 |
|
|
|
88,105 |
|
|
|
145,105 |
Shalini Sharp(2) |
|
|
16,648 |
|
|
|
- |
|
|
|
16,648 |
15
The following table sets forth the number of outstanding equity awards held by the non-employee directors who served during 2023 as of December 31, 2023:
Name |
|
Option Awards |
|
Connie Matsui |
|
|
97,076 |
Michael Dybbs, Ph.D. |
|
|
111,643 |
John Freund M.D. |
|
|
111,643 |
Heidi Hunter |
|
|
77,384 |
Joseph Lobacki |
|
|
127,988 |
James Panek |
|
|
98,000 |
Daniel Petree |
|
|
115,526 |
Jon Wigginton M.D. |
|
|
86,000 |
Shalini Sharp |
|
|
86,843 |
OUR BOARD OF DIRECTORS RECOMMENDS A VOTE “FOR” THE ELECTION OF EACH OF THE NOMINATED CLASS III DIRECTORS.
16
PROPOSAL NO. 2
RATIFICATION OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
Our Audit Committee has selected Ernst & Young LLP as our principal independent registered public accounting firm to perform the audit of our financial statements for the fiscal year ending December 31, 2024. Ernst & Young LLP audited our financial statements for the fiscal years ended December 31, 2023 and 2022. We expect that representatives of Ernst & Young LLP will be present at the Annual Meeting, will be able to make a statement if they so desire and will be available to respond to appropriate questions.
At the Annual Meeting, the stockholders are being asked to ratify the appointment of Ernst & Young LLP as our independent registered public accounting firm for the fiscal year ending December 31, 2024. Although ratification by stockholders is not required by law, our Audit Committee is submitting the selection of Ernst & Young LLP to our stockholders because we value our stockholders’ views on our independent registered public accounting firm and as a matter of good corporate governance. If this proposal does not receive the affirmative approval of a majority of the votes cast on the proposal, the Audit Committee would reconsider the appointment. Notwithstanding its selection and even if our stockholders ratify the selection, our Audit Committee, in its discretion, may appoint another independent registered public accounting firm at any time during the year if the Audit Committee believes that such a change would be in our best interests and the interests of our stockholders.
The following table presents fees for professional audit services rendered by Ernst & Young LLP for the audit of our annual financial statements for the years ended December 31, 2023 and 2022.
Principal Accountant Fees and Services
Fees Billed |
|
Fiscal Year 2023 |
|
|
Fiscal Year 2022 |
|
||
Audit fees(1) |
|
$ |
1,311,536 |
|
|
$ |
1,217,318 |
|
Audit-related fees(2) |
|
|
40,316 |
|
|
|
40,000 |
|
Tax fees(3) |
|
|
211,676 |
|
|
|
113,300 |
|
All other fees(4) |
|
— |
|
|
— |
|
||
Total fees |
|
$ |
1,563,528 |
|
|
$ |
1,370,618 |
|
Policy on Audit Committee Pre-Approval of Audit and Permissible Non-Audit Services of Independent Registered Public Accounting Firm
Our Audit Committee generally pre-approves all audit and permissible non-audit services provided by the independent registered public accounting firm. These services may include audit services, audit-related services, tax services and other services. Pre-approval is detailed as to the particular service or category of services and is generally subject to a specific budget. The independent registered public accounting firm and management are required to periodically report to the Audit Committee regarding the extent of services provided by the independent registered public accounting firm in accordance with this pre-approval, and the fees for the services performed to date. Our Audit Committee may also pre-approve particular services on a case-by-case basis. All of the services relating to the fees described in the table above were approved by our Audit Committee.
OUR BOARD OF DIRECTORS RECOMMENDS A VOTE “FOR” APPROVAL OF PROPOSAL NO. 2.
17
REPORT OF THE AUDIT COMMITTEE
The information contained in the following report of the Audit Committee is not considered to be “soliciting material,” “filed” or incorporated by reference in any past or future filing by us under the Securities Exchange Act of 1934, as amended, or the Securities Act of 1933, as amended, unless and only to the extent that we specifically incorporate it by reference.
The Audit Committee has reviewed and discussed with our management and Ernst & Young LLP our audited financial statements as of and for the year ended December 31, 2023. The Audit Committee has also discussed with Ernst & Young LLP the matters required to be discussed by the applicable requirements of the Public Company Accounting Oversight Board (United States) and the U.S. Securities and Exchange Commission.
The Audit Committee has received and reviewed the written disclosures and the letter from Ernst & Young LLP required by applicable requirements of the Public Company Accounting Oversight Board regarding the independent accountant’s communications with the Audit Committee concerning independence and has discussed with Ernst & Young LLP its independence.
Based on the review and discussions referred to above, the Audit Committee recommended to our Board of Directors that the audited financial statements as of and for the year ended December 31, 2023 be included in our Annual Report on Form 10-K for the year ended December 31, 2023 for filing with the U.S. Securities and Exchange Commission.
Submitted by the Audit Committee
Heidi Hunter, Chair
John Freund
Joseph Lobacki
18
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
The following table sets forth certain information with respect to the beneficial ownership of our common stock as of March 31, 2024, by:
each stockholder known by us to be the beneficial owner of more than 5% of our common stock;
each of our directors or director nominees;
each of our named executive officers; and
all of our directors and executive officers as a group.
Percentage ownership of our common stock is based on 62,447,713 shares of our common stock outstanding on March 31, 2024. We have determined beneficial ownership in accordance with the rules of the SEC, and thus it represents sole or shared voting or investment power with respect to our securities, and the information is not necessarily indicative of beneficial ownership for any other purpose. Unless otherwise indicated below, to our knowledge, the persons and entities named in the table have sole voting and sole investment power with respect to all shares that they beneficially owned, subject to community property laws where applicable. We have deemed all shares of common stock subject to options or other convertible securities held by that person or entity that are currently exercisable or that will become exercisable within 60 days of March 31, 2024 to be outstanding and to be beneficially owned by the person or entity holding the option for the purpose of computing the percentage ownership of that person or entity but have not treated them as outstanding for the purpose of computing the percentage ownership of any other person or entity. Unless otherwise indicated, the address of each beneficial owner listed in the table below is c/o Sutro Biopharma, Inc., 111 Oyster Point Boulevard, South San Francisco, California 94080.
|
|
Beneficial Ownership |
|||||||||||
Name of Beneficial Owner |
|
Number |
|
|
Percent |
||||||||
5% Stockholders |
|
|
|
|
|
|
|
|
|
||||
Blackrock, Inc.(1) |
|
|
5,823,922 |
|
|
|
9.3 |
|
% |
||||
Suvretta Capital Management, LLC(2) |
|
|
5,778,188 |
|
|
|
9.3 |
|
% |
||||
Adage Capital Partners GP, LLC(3) |
|
|
4,467,796 |
|
|
|
7.2 |
|
% |
||||
Integrated Core Strategies (US) LLC(4) |
|
|
3,451,825 |
|
|
|
5.5 |
|
% |
||||
BVF Inc.(5) |
|
|
3,347,946 |
|
|
|
5.4 |
|
% |
||||
Rubric Capital Management LP(6) |
|
|
3,344,810 |
|
|
|
5.4 |
|
% |
||||
Directors and Named Executive Officers: |
|
|
|
|
|
|
|
|
|
||||
Connie Matsui (7) |
|
|
94,992 |
|
|
* |
|
|
|||||
Michael Dybbs, Ph.D.(8) |
|
|
109,559 |
|
|
* |
|
|
|||||
John G. Freund, M.D.(9) |
|
|
165,886 |
|
|
* |
|
|
|||||
Heidi Hunter(10) |
|
|
71,300 |
|
|
* |
|
|
|||||
Joseph Lobacki11) |
|
|
125,904 |
|
|
* |
|
|
|||||
James Panek(12) |
|
|
95,916 |
|
|
* |
|
|
|||||
Daniel Petree(13) |
|
|
135,899 |
|
|
* |
|
|
|||||
Jon M. Wigginton, M.D.(14) |
|
|
83,916 |
|
|
* |
|
|
|||||
William J. Newell (15) |
|
|
1,726,068 |
|
|
2.7 |
|
% |
|||||
Anne Borgman(16) |
|
|
81,561 |
|
|
|
* |
|
|
||||
Hans-Peter Gerber(17) |
|
|
2,500 |
|
|
|
* |
|
|
||||
All executive officers and directors as a group (15 persons)(18) |
|
|
3,854,507 |
|
|
|
5.9 |
|
% |
||||
* Represents beneficial ownership of less than one percent.
19
20
21
EXECUTIVE OFFICERS
The following table provides information regarding our executive officers as of March 31, 2024:
Name |
|
Age |
|
Position(s) |
William J. Newell |
|
66 |
|
Chief Executive Officer and Director |
Jane Chung, RPh |
|
53 |
President and Chief Operating Officer |
|
Edward Albini |
|
66 |
|
Chief Financial Officer |
Anne Borgman, M.D. |
|
56 |
|
Chief Medical Officer |
Linda Fitzpatrick |
|
67 |
|
Chief People and Communications Officer |
Hans-Peter Gerber |
61 |
|
Chief Scientific Officer |
|
Venkatesh Srinivasan |
62 |
|
Chief Technical Operations Officer |
|
William J. Newell has served as our Chief Executive Officer and a member of our Board of Directors since January 2009. Mr. Newell’s biographical information is set forth above under the heading “Proposal No. 1 Election of Class III Directors – Continuing Directors.”
Jane Chung, RPh has served as our President and Chief Operating Officer since December 2023 and previously as our Chief Commercial Officer since August 2021. From May 2015 to August 2021, Ms. Chung served in several leadership roles at AstraZeneca, including as President and General Manager of AstraZeneca Canada, Vice President of Sales and Marketing of U.S. lmmuno-Oncology, and Senior Commercial Business Director. Prior to that, from May 2013 to May 2015, Ms. Chung served as a Regional Sales Director and Director of Sales Productivity and Effectiveness for Onyx Pharmaceuticals Inc. From October 2003 to May 2013, she served in various commercial roles for Genentech, Inc., including as Commercial Operations Manager, Division Manager and Senior Marketing Manager. Ms. Chung also serves on the Board of Directors of Viracta Therapeutics, Inc. and on non-profit boards in the science, education, and community development arenas. Ms. Chung received a B.S. in Pharmacy from St. John's University and a B.A. in Psychology from Columbia University.
Edward Albini has served as our Chief Financial Officer since January 2013. During 2012, Mr. Albini served as a consulting Chief Financial Officer for Carbylan Biosurgery, a company focused on the development and commercialization of advanced biomaterial-based joint therapies. From 2011 to 2016, Mr. Albini also served as Chief Financial Officer and Secretary for Itero Holdings, LLC, a successor entity to Itero Biopharmaceuticals, Inc., a company focused on the development and commercialization of protein therapeutics, at which Mr. Albini served as Chief Financial Officer and Senior Vice President from 2009 to 2011. Previously, Mr. Albini served as Chief Financial Officer of Novacea, Inc. and Lynx Therapeutics, Inc., both biopharmaceutical companies. Mr. Albini received a B.S.C. in Accounting from Santa Clara University and an M.B.A. from the Walter A. Haas School of Business at the University of California, Berkeley. Mr. Albini is also a certified public accountant (inactive status) in California.
Anne Borgman, M.D., has served as our Chief Medical Officer since February 2023. Dr. Borgman previously served as Principal, AEB Hematology Oncology Development Consulting from November 2021 to February 2023. From July 2019 to November 2021, Dr. Borgman served as Vice President and Global Therapeutic Area Lead, Hematology-Oncology, at Jazz Pharmaceuticals, Inc. From November 2012 to July 2019, Dr. Borgman served as Vice President, Clinical Research & Development at Exelixis, Inc. Prior to that, Dr. Borgman was Chief Medical Officer and Vice President of Hana Biosciences (now Talon Therapeutics, Inc.), Associate Chief Medical Officer at KaloBios Pharmaceuticals, Inc., and Global Project Head, oncology, at Abbott Laboratories. From 2012 to 2019, Dr. Borgman was a Consulting Associate Professor at Stanford University School of Medicine’s Stem Cell Transplant & Cell Biology program, and from 2001 to 2010, a Clinical Associate at University of Chicago’s Department of Pediatrics, Division of Oncology and Stem Cell Research. Dr. Borgman currently serves on the Board of Directors at Curis, Inc., NextCure, Inc., and NiKang Therapeutics, Inc. Dr. Borgman earned her B.S. in Biochemistry from University of Illinois and her M.D. from Loyola University of Chicago’s Stritch School of Medicine, before completing her residency in Pediatrics at Texas Children’s Hospital and Baylor College of Medicine, and her fellowship in Pediatric Hematology-Oncology at UCLA School of Medicine.
22
Linda Fitzpatrick has served as our Chief People and Communications Officer since August 2018. From January 2008 to August 2018, Ms. Fitzpatrick served as our VP of Human Resources and Communications in the capacity of Senior Advisor. In addition to her strategic consulting practice, she co-founded Parallax Venture Partners, an early stage health care venture fund in April 2002. From October 1992 to March 2002, Ms. Fitzpatrick served as Vice President of Human Resources, Corporate Communications and Operations for Gilead Sciences, Inc. and from February 1985 to September 1992 she served as Director of Investor Relations and Director of Compensation, Benefits and Systems for Genentech, Inc. in addition to heading the human resources and corporate communications strategy for a variety of publicly held biotechnology companies. Ms. Fitzpatrick also serves on a variety of non-profit boards, including board chair roles, in the science, education and community development arenas. Ms. Fitzpatrick received a B.A. in Psychology and Sociology from San Francisco State University.
Hans-Peter Gerber, Ph.D. has served as our Chief Scientific Officer since September 2023. From April 2022 to August 2023, Dr. Gerber served as Chief Scientific Officer and Co-founder of Codeable Therapeutics, an ADC startup company. From April 2018 to March 2022, Dr. Gerber served as Chief Scientific Officer and Senior Vice President of 3T Biosciences, Inc. From November 2016 to March 2018, Dr. Gerber served as President and Chief Scientific Officer of Maverick Therapeutics, Inc., which was acquired by Takeda Pharmaceutical Company in 2021. Prior to his service at Maverick, Dr. Gerber held leadership positions at several top biopharmaceutical companies, including Genentech, Seattle Genetics, and Pfizer, where he led the R&D group as Chief Scientific Officer. Dr. Gerber earned his M.S. in Biochemistry and his Ph.D in Molecular Biology from the University of Zurich, Switzerland.
Venkatesh Srinivasan, Ph.D. has served as our Chief Technical Officer since May 2023. From April 2022 to May 2023, Dr. Srinivasan served as our Senior Vice President, Process, Analytical and Formulation Development. Prior to that, from March 2011 to March 2022, Dr. Srinivasan worked at Bayer AG, where he was most recently the Vice President of Global Manufacturing Sciences and Technology. Dr. Srinivasan also co-founded GlycoRx Partners, LLC, a company focused on developing novel, half-life extended pharmaceutical proteins and peptides based on a unique glycoengineering platform, where he was a partner from 2009 to 2011. Prior to that, Dr. Srinivasan worked for Phyton Biotech, LLC where he served as Senior Director, Bioprocess Development and Director, Business Development. Dr. Srinivasan received his B.S. in Chemical Engineering from Indian Institute of Technology, Chennai, and his Ph.D. in Chemical Engineering from the State University of New York. He also held post-doctoral appointments at the School of Engineering, Cornell University, and at the Antibody Engineering Laboratory, University of California, Davis.
EXECUTIVE COMPENSATION
Overview and Narrative
This overview and narrative (“Compensation Disclosure”) describes the key elements of our executive compensation program and compensation decisions for our named executive officers, or NEOs, for 2023. This Compensation Disclosure is intended to be read in conjunction with the tables that immediately follow this section, which provide additional compensation information for our named executive officers. We are a “smaller reporting company” as defined under Rule 405 of the Securities Act of 1933, as amended, and, as such, we are not required to provide the additional disclosures included in this Compensation Disclosure. However, we believe the additional narrative disclosure with respect to our executive compensation program will provide our stockholders with further information regarding our company and our executive compensation program and practices and therefore will assist in their consideration of Proposal No. 3, the non-binding vote with respect to named executive officer compensation.
Our executive compensation programs are designed to attract, motivate and retain qualified and talented executives, incentivize them to achieve our business objectives, and reward them for superior short- and long-term performance and results. The Compensation Committee of the Board of Directors, with input from its independent compensation consultant, oversees these programs and determines compensation for our NEOs.
Our 2023 NEOs were:
NEO |
|
Class |
William J. Newell |
|
Chief Executive Officer |
Anne Borgman, M.D. |
|
Chief Medical Officer |
Hans-Peter Gerber, Ph.D. |
|
Chief Scientific Officer |
Executive Summary
We are a clinical-stage oncology company developing site-specific and novel-format antibody drug conjugates, or ADCs, enabled by our proprietary integrated cell-free protein synthesis platform, XpressCF®, and our site-specific conjugation platform, XpressCF+®.
23
We aim to design and develop therapeutics using the most relevant and potent modalities, including ADCs, bispecific ADCs, immunostimulatory ADCs, or iADCs, dual conjugate ADCs, or ADC2s, and cytokine derivatives. Our molecules are directed primarily against clinically validated targets where the current standard of care is suboptimal. We believe that our platform allows us to accelerate the discovery and development of potential first-in-class and/or best-in-class molecules by enabling the rapid and systematic evaluation of protein structure-activity relationships to create optimized homogeneous product candidates. Our mission is to transform the lives of patients by creating medicines with improved therapeutic profiles for areas of unmet need.
Our NEO compensation program in 2023 was designed to be aligned with industry practices and align executive compensation with our performance, with an emphasis on long-term equity compensation in the form of stock options and restricted stock units, or RSUs.
Business Highlights
Since 2009, our leadership team has built the Company focusing on our pipeline and platform, delivering on key milestones, furthering our capabilities with strong partner relationships, building the organization, and ensuring the financial strength of the Company.
We have a broad pipeline with multiple clinical stage assets deployed across multiple oncology indications. Based on our proprietary XpressCF® and XpressCF+® platforms, we have also entered into multi-target, product-focused collaborations with leaders in the field of oncology. We have advanced multiple ongoing global trials for our lead product candidate and continue to support the development of partnered product candidates in clinical trials and expect to advance additional potential product candidates towards the clinic.
Key 2023 achievements reflected strong scientific, clinical, and business performance, which we believe have the opportunity to create long-term value for our stockholders. Among our 2023 results were the following achievements:
24
Compensation Practices and Governance Highlights |
|
Pay for Performance |
Significant link between the compensation of our NEOs and the achievement of our short- and long-term business objectives through annual cash incentives that are tied to key scientific, clinical, development, manufacturing and business milestones and with long-term equity compensation that rewards creation of stockholder value through stock options and RSUs |
Stockholder Alignment |
Alignment of the interests of our NEOs with those of our stockholders through the use of long-term equity incentives, the value of which is dependent on our stock price performance |
Compensation Governance |
Independent directors on the Compensation Committee Compensation Committee meets regularly in executive session without management present Independent compensation consultant, Frederic W. Cook & Co., reports directly to the Compensation Committee Compensation Committee regularly considers risks related to compensation policies and practices |
Change in Control Provisions |
Change in control payments contingent upon “double-trigger” No tax gross-ups on severance or change in control benefits |
Post-termination/Retirement Benefits |
No post-termination retirement or pension benefits |
Prohibition on Hedging, Margin Loans and Pledging |
Prohibit hedging, purchases on margin, and pledging of our common stock by all employees and directors |
Clawback Policy |
Maintain a compensation recovery policy to ensure accountability |
Stockholder Advisory Vote
At our annual meeting of stockholders on June 6, 2024, we will again conduct a non-binding stockholder advisory vote on the compensation of our named executive officers (commonly known as a “Say-on-Pay” vote). We conducted a Say-on-Pay vote in 2023, in which the stockholders approved on an advisory basis the compensation of our named executive officers with a 80.7% support rate. We value the opinions of our stockholders, and the Compensation Committee and the Board of Directors considered the outcome of the advisory vote, and will continue to do so in the future, including the vote which will take place at the 2024 annual meeting, when making compensation decisions for the NEOs.
Compensation Philosophy, Objectives and Development
Our 2023 compensation strategy emphasizes pay for performance by using both performance-based annual cash incentives and long-term equity awards in the form of stock options and RSUs. Stock options are utilized because they only provide value if the stock price increases. RSUs are also used to retain talent, align with long-term value (both increases and reductions), and because RSUs are less dilutive than stock options. Our stock options generally vest monthly over four years; with a one-year cliff vesting associated with a new hire grant, and our RSUs generally vest annually over four years (with 25% vesting annually), to align with long-term value creation as well as our clinical development and commercialization timeline.
Consistent with our pay-for-performance philosophy, and the long product development life cycles in the biopharmaceuticals industry, the Compensation Committee links the compensation of our executive officers to performance by emphasizing equity compensation opportunities for long-term performance and cash incentives for near-term goal alignment. Thus, the total compensation provided to our executive officers will vary from year to year and will vary between executive officers based on corporate performance, including performance against annual goals that are pre-established by the Compensation Committee, as well as individual performance. The Compensation Committee’s 2023 compensation decisions reflected, among other things, the level of goal achievement related to the potential for long-term value creation from our platform and clinical, manufacturing and business achievements. The Compensation
25
Committee will continue to consider scientific and business achievements and market capitalization, among the many factors considered, when making compensation decisions.
Our NEOs are also provided market-competitive health and welfare benefits, and as described below, they may be entitled to receive additional benefits if certain criteria are met at termination of employment.
We will continue to refine the design of our compensation program and implement one that is appropriate for our company given our business, industry, growth, and other factors.
Program Development and Role of Compensation Committee, Compensation Consultant and Management
Role of Compensation Committee. Our Compensation Committee is responsible for overseeing the total compensation of our executive officers, including the underlying philosophy and related policies. As such, the Compensation Committee designs, implements, reviews and approves all compensation for our CEO and our other executive officers. From time to time, and in conjunction with our Compensation Committee, the independent members of our Board of Directors, including our Chairperson, may also be involved in setting the compensation of our CEO and other executive officers and determining the corporate objectives upon which our short-term incentives are based and assessed. Our Compensation Committee’s decisions and recommendations regarding executive compensation are based on the Compensation Committee’s assessment of the performance of our company and each individual executive officer, as well as other factors, such as prevailing industry trends, retention, and the competitive market for executive talent.
The Compensation Committee consults with its compensation consultant, legal counsel and other advisors in designing our compensation program, including in evaluating the competitiveness of individual compensation and in relation to our performance goals. The Compensation Committee also considers peer company data and factors such as the past, current and expected contributions of each NEO, our corporate performance and strategic focus, global economic conditions, the mix of compensation that would be most appropriate for each NEO, and such officer’s particular responsibilities, experience, level of accountability and decision authority.
Role of CEO and Management. As part of the process for setting the compensation of our NEOs, our Chief Executive Officer, working with our Chief People and Communications Officer, provides the Compensation Committee with his performance assessments of the company and of the individual NEOs and recommends to the Compensation Committee base salaries, target annual cash incentives (as a percentage of base salary), annual cash incentive payouts (actual incentive paid based on performance against goals), and stock-based compensation for our NEOs (other than for himself). The Compensation Committee considers our Chief Executive Officer’s input and can accept, reject or modify these recommendations in its discretion.
The Compensation Committee meets in executive session without management. Various members of management may attend Committee meetings, and they and other employees, as well as outside advisors or consultants, may be invited by the Compensation Committee to make presentations, provide financial, competitive market, or other background information or advice. None of our NEOs were present during the Compensation Committee’s determinations regarding his or her own compensation.
Role of Independent Compensation Consultant & Market Data. The Compensation Committee has retained FW Cook as its compensation consultant. FW Cook reports directly to the Compensation Committee and takes its direction from the Chair of the Compensation Committee, working with management on select issues under the Compensation Committee’s oversight. The Compensation Committee retained FW Cook in 2023 to provide data, context, and advice regarding executive officer compensation and our peer group, and to assist with compensation risk assessments.
Peer Groups Used in Program Development and Compensation Decisions
Our Compensation Committee considers peer data as one factor in its overall compensation analysis and for purposes of assessing the competitiveness of the executive compensation program. An individual NEO may earn more or less than the peer group median depending on factors described below under the heading “2023 Compensation Decisions,” including the individual’s experience, role, and past and expected future performance.
Fiscal 2023 Peer Group
In the third quarter of 2022, the Compensation Committee reviewed and updated our peer group to include the group of companies set forth below based on, among other considerations, objective size criteria, including industry, market capitalization, headcount, pipeline, location, scope of operations and commercial stage. We refer to this peer group of 19 companies as the “2023 Peer Group.” When this peer group was approved by the Compensation Committee to inform 2023 compensation, these companies had July 29, 2022 market capitalizations of between $159 million and $1.9 billion, and our $275 million market capitalization on this date was within the peer range.
26
Fiscal 2023 Peer Group |
||||
Alaunos Therapeutics |
|
Clovis Oncology |
|
Kura Oncology |
Alector |
|
G1 Therapeutics |
|
MacroGenics |
Allogene Therapeutics |
|
Gossamer Bio |
|
Mersana Therapeutics |
Anaptys Bio |
|
Gritstone Bio |
|
Replimune Group |
Atara Biotherapeutics |
|
ImmunoGen |
|
Sangamo Therapeutics |
Arcus Biosciences |
|
Jounce Therapeutics |
|
Zymeworks |
ChemoCentryx |
|
|
|
|
The following companies with larger market capitalization than Sutro were removed from the 2023 Peer Group to better-align the group’s overall market capitalization with Sutro’s: Cytokinetics, Denali Therapeutics, and Fate Therapeutics. Odonate Therapeutics was also removed because it was delisted. Five companies that fit the Compensation Committee’s desired criteria and had market capitalization on July 29, 2022 ranging from $159 million to $841 million were added to the 2023 Peer Group: Alector, AnaptysBio, Gossamer Bio, Gritstone Bio, and Jounce Therapeutics.
Compensation Consultant Conflict of Interest Analysis
The Compensation Committee has determined that the work of FW Cook and the individual compensation advisors employed by FW Cook does not create any conflict of interest. In making that determination, the Compensation Committee took into consideration the following factors: (i) the provision of other services to us by FW Cook; (ii) the amount of fees we paid FW Cook as a percentage of FW Cook’s total revenue; (iii) FW Cook’s policies and procedures that are designed to prevent conflicts of interest; (iv) any business or personal relationship of FW Cook or the individual compensation advisors employed by FW Cook with a Sutro executive officer; (v) any business or personal relationship of the individual compensation advisors with any member of the Compensation Committee; and (vi) any Sutro stock owned by FW Cook or the individual compensation advisors employed by the consultant. During 2023, we paid FW Cook fees that constituted less than 1% of FW Cook’s total revenue.
2023 Compensation Decisions
Summary
Most 2023 compensation decisions were made in the first quarter of 2023 and were significantly influenced by our scientific and business achievements together with the competitive market for experienced talent. Our 2023 compensation decisions were based on a review of individual performance and potential; achievement of the Company’s corporate goals, including advancing our research and clinical pipelines and financial performance; and a market analysis of competitive compensation based on our 2023 peer group.
Following are highlights of the 2023 compensation decisions:
Base Salary
The purpose of base salary is to provide fixed compensation to attract, retain and motivate executives with the qualifications desired for the individual position. The base salary for our NEOs is influenced by a number of factors, including the individual’s position, scope of responsibilities, breadth and depth of experience, performance to date, expected future contribution, and the overall mix of base salary, performance-based cash incentives and equity compensation.
27
The 2023 base salaries for our NEOs were as follows:
NEO |
|
2022 Annual Base Salary |
|
2023 Annual Base Salary |
|
Increase (%) |
|||
William Newell |
|
$ |
662,500 |
|
$ |
686,667 |
|
|
3.6% |
Anne Borgman, M.D. |
|
|
- |
|
|
500,000(1) |
|
|
N/A |
Hans-Peter Gerber, Ph.D. |
|
|
- |
|
|
500,000(2) |
|
|
N/A |
Performance-Based Cash Incentives
All of our NEOs are eligible for annual performance-based cash incentive bonuses, which are designed to reward achievement of certain annual corporate goals that the Company believes will ultimately drive stockholder value. Each performance-based cash incentive bonus opportunity is expressed as a target percentage of annual base salary. Mr. Newell’s target performance-based cash bonus was 60% of his annual base salary, Dr. Borgman’s target was 40% of her annual base salary and Dr. Gerber’s target was 40% of his annual base salary. The incentive target as a % of salary for Mr. Newell remained the same in 2023 as in 2022; Dr. Borgman and Dr. Gerber joined the Company in 2023.
No amount of incentive award was guaranteed. Each NEO’s incentive award payout was based on the level of achievement of corporate goals determined by the Compensation Committee in early 2023, and for NEOs other than our CEO, the participant’s role in such goal achievement and the weighting of the goals, with the Compensation Committee retaining discretion to adjust or modify actual awards. The Compensation Committee did not exercise this discretion.
The corporate performance goals for the fiscal year 2023 cash incentive bonus program were determined by the Compensation Committee and included certain developmental, research, financial, and operational milestones, as discussed below. These milestones were selected to drive appreciation of value in the Company, including maintaining timelines for development of the Company’s and the Company’s partners’ clinical and preclinical product candidates, successful conclusion of licensing and/or collaboration transactions, expansion and development of the Company’s manufacturing activities, including through third party contract development and manufacturing organizations, maintenance of an appropriate cash position and runway, and maintaining Company culture and employee morale. Each of these goals pertain to confidential company development and business plans, the disclosure of which in any additional granularity would result in competitive harm to the company. The Compensation Committee believed that each of these goals would be challenging to achieve.
In early 2024, the Compensation Committee assessed our performance against the 2023 corporate goals, using both quantitative measurement and qualitative evaluation. The Compensation Committee determined that the Company had achieved its corporate goals at an overall level of 90% of target due to partial achievement with respect to business development and dealmaking goals, proprietary pipeline progression goals, and financial performance goals and overachievement in goals related to partnered pipeline progression. The 2023 corporate goals, the weighting of such goals, and the facts the Compensation Committee considered in determining the achievement of such goals are set forth below.
In assessing the 2023 corporate goals, the Compensation Committee considered achievement in the following categories with the target weightings as noted:
The Compensation Committee determined that the Company fully achieved its goals with respect to Pivotal Trials and Commercial Readiness, focusing primarily on the development of the Company’s lead wholly-owned asset, luvelta. In particular, the Company initiated a registrational Phase 2/3 study of luvelta for treatment of platinum resistant ovarian cancer in the first half of 2023 and met its goals with respect to patient enrollment. In addition, the Company filed an IND to assess luvelta in treatment of refractory/relapsed CBF/GLIS AML in a pediatric patient population and defined a go-forward clinical development plan in this indication. Sutro also
28
generated intriguing preliminary data in the Phase 1 dose expansion study of luvelta for the treatment of endometrial cancer and in the Phase 1 study of the combination of luvelta and bevacizumab for the treatment of patients with ovarian cancer, suggesting the potential of luvelta to treat these indications. Finally, the Company defined a clinical development strategy to assess luvelta for the treatment of non-small cell lung cancer and made substantial progress on its execution.
The Compensation Committee determined that the Company partially achieved its goals with respect to proprietary pipeline progression, focusing on the progress made to bring STRO-003 and STRO-004 to the clinic. While the Company is on track to file an IND in connection with STRO-003 in 2024, that IND was delayed from its initial proposed timing, leading to partial achievement of the goal. STRO-004 was nominated as a development candidate in 2023 and is on track to begin clinical studies in 2025 as projected, meeting this part of the goal.
With respect to the goal relating to partnered program progression, the Compensation Committee determined that the Company substantially overachieved its goals. Our agreements with our collaboration partners do not permit us to disclose the milestones achieved in these relationships because of applicable confidentiality obligations.
The Compensation Committee further determined that the Company partially achieved its goals with respect to new dealmaking, noting that the Company did not execute a luvelta partnering or financing transaction or identify a strategic manufacturing partner. Underachievement in dealmaking was mitigated by the successful royalty monetization transaction executed with Blackstone and by Vaxcyte’s exercise of the manufacturing rights option negotiated in December 2022.
The Compensation Committee determined that the Company fully achieved its goals in connection with placing manufacturing on track for the next stage. Achievement in this area resulted from significant and meaningful progress in the technology transfer of the XtractCF® and other reagents manufacturing processes to our third party contract development and manufacturing organizations, or CDMOs. Meaningful progress was also made in transferring the process for manufacturing antibody intermediates used to make the Company’s clinical products to our CDMO. We also made important progress in defining our regulatory CMC strategy for luvelta.
With regard to the Company’s financial performance, the Compensation Committee determined that the Company partially achieved in this area. In particular, the Company ended the year with a cash and investments balance, including the value of Vaxcyte common stock held by Sutro, sufficient to fund activities into the second half of 2025, while delivering upon short and long-term strategic objectives in accordance with the Company’s goals.
The Compensation Committee determined that the Company fully achieved with respect to its commitment to execution, culture, talent and team. In particular, the Company hired and integrated a significant number of new employees, while maintaining a turnover rate lower than industry standard. The Company further enhanced its company-wide learning and development programs, continued to build upon a comprehensive internship program (focusing on individuals who are first in their family to pursue higher education), and leveraged its Diversity, Equity, Inclusion and Belonging Council to support both internal and external development initiatives. In recognition of its efforts in this area, the Company was awarded second position in BioSpace’s list of best places to work for employers with fewer than 1,000 employees.
In summary, based on substantial overachievement in one goal, full achievement in three goals, and partial achievement in three goals, the Compensation Committee determined an overall goal achievement for the Company in 2023 of 90%.
The Compensation Committee awarded Mr. Newell a bonus equal to our corporate performance for the year because it determined that his performance as CEO aligns directly with our corporate performance. The Compensation Committee determined that the bonus achievement for each of the other NEOs is based on both our corporate performance and its assessment of each NEO’s individual contributions towards our corporate performance. The amount of each NEO’s earned cash incentive award at 90% achievement, and their target award, are reflected below:
NEO |
|
Target Award |
|
Actual Award |
||
William Newell |
|
$ |
414,000 |
|
$ |
372,600 |
Anne Borgman, M.D. |
|
|
200,000 |
|
|
180,000 |
Hans-Peter Gerber, Ph.D. |
|
|
200,000 |
|
|
180,000 |
29
Sign-On Bonuses.
We agreed to pay each of Drs. Borgman and Gerber a signing bonus of $350,000 as an inducement to commence employment with us. Each of Drs. Borgman and Gerber received $250,000 on the first payroll period following their service start date. Drs. Borgman and Gerber will be paid the remaining $100,000 on their one-year anniversary subject to their continued service to us. The signing bonus is subject to repayment in the event either Dr. Borgman or Dr. Gerber resigns or is terminated for “cause” before the two-year anniversary of their employment start date.
Equity Compensation
General. We believe that equity grants provide our NEOs with the opportunity to share in increases, if any, in the value of our common stock, reinforce a long-term interest in our corporate performance, and directly motivate our NEOs to maximize long-term stockholder value. The potential realized value of certain grants depends on our stock performance and all of our equity grants utilize vesting to encourage our NEOs to continue working for us for the long term.
The Compensation Committee determines the size and type of equity awards after evaluating various factors applicable at the time of each such grant in their totality, which has included, among other things: the particular NEO’s role and responsibilities and the Compensation Committee’s view of the officer’s individual performance; the prior equity awards granted to such individual; retentive value of prior awards; our corporate performance; the value of equity grants; comparative peer data provided by its compensation consultant; and dilution to our stockholders.
All grants to executive officers require the approval of the Compensation Committee.
2023 Equity Grants. The Compensation Committee granted our NEOs equity compensation in the first quarter of 2023, or at the time of their later commencement of employment, in the form of stock options and RSUs, consistent with our compensation philosophy that our NEOs should have a significant proportion of their total compensation tied to our performance, to encourage retention, and to align their long-term compensation with the interests of our stockholders. The Compensation Committee felt that providing equity opportunities for our NEOs with an equity mix that included RSUs would balance alignment of executives with stockholder growth and support retention during a multi-year product development and commercial cycle. Our Compensation Committee strives to maintain a compensation program that is tied to performance and is competitive with market practices.
The Compensation Committee determined the size of the equity grants based on several factors, including:
The stock option and RSU grants to each of our NEOs are reflected in the table below. The grant value for the CEO was meaningfully lower than both his 2022 equity compensation and the median of grant value benchmarks from the 2023 Peer Group that were reviewed by the Compensation Committee in late 2022 and early 2023. The stock option and RSU grants to the other two NEOs were new hire grants as an inducement to commence employment with the Company.
|
|
2023 Stock |
|
2023 Stock |
|
2023 RSUs |
|
2023 RSUs |
||
NEO |
Options (#) |
Options ($)(1) |
(#) |
($)(1) |
||||||
William Newell |
|
125,000 |
|
|
520,663 |
|
95,000 |
|
|
550,050 |
Anne Borgman, M.D. |
|
175,000 |
|
|
676,568 |
|
150,000 |
|
|
810,000 |
Hans-Peter Gerber, Ph.D. |
|
175,000 |
|
|
485,643 |
|
150,000 |
|
|
565,500 |
30
2023 Stock Options. The stock options granted to Mr. Newell in 2023 vest equally in monthly installments over four years. The new hire stock options granted to Ms. Borgman and Mr. Gerber vests with respect to 25% of the shares subject to the options on the first anniversary of the grant date, and the remainder of the shares vesting monthly over the following three years in equal installments.
2023 Restricted Stock Units. The RSUs granted to our NEOs in 2023 vest equally in annual installments over four years.
Benefits Programs
Our employee benefit programs, including our 401(k) plan, employee stock purchase plan, and health and welfare programs, including health savings accounts and flexible spending arrangements, are designed to provide a competitive level of benefits to our employees generally, including our executive officers, and their families. We adjust our employee benefit programs as needed, based upon regular monitoring of applicable laws and practices and the competitive market. Our executive officers are eligible to participate in the same employee benefit plans and programs, and on the same terms and conditions, as all other U.S. full-time employees.
Perquisites and Other Personal Benefits
Currently, we do not view perquisites or other personal benefits as a component of our executive compensation program. Accordingly, we do not generally provide perquisites to our executive team. In the future, we may provide perquisites or other personal benefits in limited circumstances, such as where we believe it is appropriate to assist an individual executive in the performance of his or her duties, to make our executive team more efficient and effective and for recruitment, motivation or retention purposes. All future practices with respect to perquisites or other personal benefits will be subject to review and approval by the Compensation Committee.
Employment Agreements and Severance and Change in Control Benefits
We have entered into written employment agreements with each of our NEOs that set forth the terms of their employment, including initial base salaries and eligibility to earn a bonus, as well as standard confidentiality and invention assignment agreements. Each of our named executive officers is employed “at will.” These arrangements are further described under the section below titled “Executive Compensation Tables.”
Our NEOs are entitled to certain severance and change in control benefits under the terms of our executive severance and change in control plan, or Severance Plan. Below is a summary of potential post-termination compensation for our NEOs. We provide these benefits because the Compensation Committee determined that it was appropriate to provide our NEOs with severance compensation if their employment is terminated under certain circumstances. The Compensation Committee believes that the severance benefits are an important element of the NEOs’ competitive pay packages, that they serve important retention and motivation purposes and that such severance benefits, including generally requiring a release of claims against us as a condition to receiving any severance benefits, are best market practice and are in the best interest of the Company and its stockholders.
Severance Benefits
On March 17, 2021, we adopted an executive severance and change in control plan, or Severance Plan, for each of our executives with the title of vice president (or equivalent) or above, including all of our NEOs. Under the Severance Plan, if we terminate an executive other than for cause or if the executive resigns for good reason, each as defined in the Severance Plan, the executive will receive: (i) cash severance payments equal to 18 months of his or her annual base salary for our Chief Executive Officer, 15 months for our other chief executives (including the non-CEO NEOs and those chief executives with an additional title) and 9 months for our senior vice presidents and vice presidents; (ii) a pro rata portion of his or her bonus based on actual achievement of applicable metrics for the year; (iii) payment of COBRA premiums for continued medical coverage for up to 18 months for our Chief Executive Officer, 15 months for our other chief executives and 9 months for our senior vice presidents and vice presidents; and (iv) accelerated vesting on outstanding time-based stock options and restricted stock units that vest within 18 months for our Chief Executive Officer, 15 months for our other chief executives and 9 months for our senior vice presidents and vice presidents (any performance-based equity awards will only be accelerated according to the terms of such awards).
In the event an executive’s employment terminates without cause or the executive resigns for good reason and such termination occurs on or within 18 months following a change in control, as defined in the Severance Plan, the executive will receive: (i) cash severance payments equal to 18 months of his or her annual base salary for our Chief Executive Officer, 15 months for our other chief executives and 9 months for our senior vice presidents and vice presidents; (ii) a pro rata portion of his or her target bonus for the year; (iii) cash severance payments equal to his or her target bonus for the year multiplied by 1.5 for our Chief Executive Officer, 1.25 for our other chief executives and 0.75 for our senior vice presidents and vice presidents; (iv) payment of COBRA premiums for continued medical coverage for up to 18 months for our Chief Executive Officer, 15 months for our other chief executives and 9 months for our senior vice presidents and vice presidents; and (v) full accelerated vesting on all outstanding stock options and restricted stock units (any performance-based equity awards will only be accelerated according to the terms of such awards).
31
As a condition to receiving these severance benefits, the executive is required to execute a release of claims agreement in favor of us. The Severance Plan continues in effect for three years and shall automatically renew for successive three-year periods thereafter, unless we give notice of non-renewal prior to such renewal date. Although we have the right to amend or terminate the Severance Plan, we may not do so in any manner that diminishes any benefits being paid to an executive at the time of such amendment or termination.
Tax & Accounting Considerations
We take into account the tax effects of various forms of compensation and the potential for excise taxes to be imposed on our executive officers. There are various provisions of the Code that we consider in determining compensation, including the following:
Section 162(m). Section 162(m) of the Internal Revenue Code generally disallows a tax deduction for compensation in excess of $1 million paid to “covered employees,” which include our current NEOs. As a result, we expect that compensation awarded to our NEOs will not be deductible to the extent it is in excess of the $1 million threshold. In determining the form and amount of compensation for our NEOs, the compensation committee will continue to consider all elements of the cost of such compensation, including Section 162(m). While the compensation committee considers the deductibility of awards as one factor in determining executive compensation, the compensation committee also looks at other factors in making its decisions and retains the flexibility to award compensation that it determines to be consistent with the goals of our executive compensation program even if the awards are not deductible by us for tax purposes.
Sections 280G and 4999. Any payment or benefit provided to executive officers in connection with a change-in-control transaction may be subject to an excise tax under Section 4999 of the Code. These payments also may not be eligible for a company tax deduction pursuant to Section 280G of the Code. If any of these payments or benefits are subject to the excise tax, they may be reduced to provide the individual with the best after-tax result. The individual will receive a reduced amount so that the excise tax is not triggered, or the individual will receive the full amount of the payments and benefits and then be liable for any excise tax. We have not agreed and are not otherwise obligated to provide any named executive officer with such a “gross-up” or other reimbursement.
We account for stock compensation in accordance with the authoritative guidance set forth in FASB ASC Topic 718, which requires companies to measure and recognize the compensation expense for all share-based awards made to employees and directors over the period during which the award recipient is required to perform services in exchange for the award. We determine both the grant date fair value and the service period based on applicable accounting standards. This calculation is performed for accounting purposes and reported in the compensation tables included in this proxy statement as applicable.
Additional Executive Compensation Practices, Policies and Procedures
Prohibition of Hedging and Pledging. We prohibit our NEOs (and other employees) and non-employee directors from engaging in hedging or monetization transactions involving our securities or contributing our securities to exchange funds that could be interpreted as having the effect of hedging in Company securities.
Compensation Recovery Policy
On November 12, 2023, our Board of Directors adopted a compensation recovery policy (the “Compensation Recovery Policy”) intended to comply with applicable SEC rules and Nasdaq listing standards. The Compensation Recovery Policy is administered by our Compensation Committee (in such capacity, the “Administrator”) and enables us to recover from current and former officers, and such additional employees as may be identified by the Administrator from time to time, incentive-based compensation, as defined in the Compensation Recovery Policy, in the event of an accounting restatement resulting from material noncompliance with any financial reporting requirements under federal securities laws. For more information, see the full text of our Compensation Recovery Policy, which is filed as an exhibit to our Annual Report.
Compensation Policies and Practices as they relate to Risk Management
The Compensation Committee has reviewed our executive and employee compensation programs and does not believe that our compensation policies and practices encourage undue or inappropriate risk taking or create risks that are reasonably likely to have a material adverse effect on us. The reasons for the Compensation Committee’s determination include the following:
32
33
EXECUTIVE COMPENSATION TABLES
Summary Compensation Table
The following table presents summary information regarding the total compensation for services rendered in all capacities that was earned by our named executive officers during the years ended December 31, 2023 and 2022, as applicable.
Name and |
|
|
|
|
|
|
|
|
|
|
|
Non-Equity |
|
|
|
|
Principal |
|
|
|
Option |
Stock |
Incentive Plan |
All Other |
|
||||||||
Position |
Year |
Salary($) |
Bonus($) |
Awards($)(1) |
Awards($)(1) |
Compensation($)(2) |
Compensation($) |
Total($) |
||||||||
William J. Newell |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Chief Executive |
|
2023 |
|
686,667 |
|
- |
|
520,663 |
|
550,050 |
|
372,600 |
|
- |
|
2,129,979 |
Officer |
|
2022 |
|
662,500 |
|
- |
|
948,972 |
|
1,017,165 |
|
402,000 |
|
8,267(3) |
|
3,038,904 |
Anne Borgman, M.D. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Chief Medical |
|
2023 |
|
418,982(4) |
|
250,000(5) |
|
676,568 |
|
810,000 |
|
180,000 |
|
- |
|
2,335,549 |
Officer |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Hans-Peter Gerber |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Chief Scientific |
|
2023 |
|
145,833(4) |
|
250,000(5) |
|
485,643 |
|
565,500 |
|
180,000 |
|
- |
|
1,626,976 |
Officer |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
34
Offer Letters with NEOs
We have entered into written employment agreements with each of our NEOs that set forth the terms of their employment, including initial base salaries and eligibility to earn a bonus, as well as standard confidentiality and invention assignment agreements. Each of our named executive officers is employed “at will.” The offer letters for Mr. Newell also included certain severance protections, which severance protections have been superseded by the Severance Plan described above in the section entitled “—Employment Agreement and Severance and Change in Control Benefits –Severance Benefits.”
Mr. Newell’s Employment Offer Letter
Mr. Newell is party to an offer letter with us dated December 29, 2008 pursuant to which he serves as our Chief Executive Officer. The terms and conditions of his offer letter provide for an annual base salary, and eligibility for an annual bonus, health insurance and other benefits, all subject to adjustment from time to time. Mr. Newell is an at-will employee. Mr. Newell participates in the Severance Plan described above in the section entitled “—Employment Agreement and Severance and Change in Control Benefits –Severance Benefits.”
Dr. Borgman’s Employment Offer Letter
Dr. Borgman is party to an offer letter with us dated January 18, 2023 pursuant to which she serves as our Chief Medical Officer. The terms and conditions of her offer letter provide for an annual base salary, and eligibility for an annual bonus, health insurance and other benefits, all subject to adjustment from time to time. Dr. Borgman is an at-will employee. Dr. Borgman participates in the Severance Plan described above in the section entitled “—Employment Agreement and Severance and Change in Control Benefits –Severance Benefits.”
Dr. Gerber’s Employment Offer Letter
Dr. Gerber is party to an offer letter with us dated August 28, 2023 pursuant to which he serves as our Chief Scientific Officer. The terms and conditions of his offer letter provide for an annual base salary, and eligibility for an annual bonus, health insurance and other benefits, all subject to adjustment from time to time. Dr. Gerber is an at-will employee. Dr. Gerber participates in the Severance Plan described above in the section entitled “—Employment Agreement and Severance and Change in Control Benefits –Severance Benefits.”
35
2023 Outstanding Equity Awards at Fiscal Year-End Table
The following table presents, for each of our named executive officers, information regarding outstanding stock options and restricted stock units held as of December 31, 2023.
|
|
Option Awards |
|
Stock Awards |
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Number |
|
Market |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
of Units |
|
Value of |
|
|
|
|
|
Number of |
|
Number of |
|
|
|
|
|
|
|
|
of Stock |
|
Units of |
|
|
|
|
|
Securities |
|
Securities |
|
|
|
|
|
|
|
|
That |
|
Stock |
|
|
|
|
|
Underlying |
|
Underlying |
|
Option |
|
|
|
|
|
|
Have |
|
That |
|
|
|
|
|
Unexercised |
|
Unexercised |
|
Exercise |
|
Option |
|
|
|
|
Not |
|
Have Not |
|
|
|
Grant |
|
Options (#) |
|
Options (#) |
|
Price |
|
Expiration |
|
Grant |
|
Vested |
|
Vested |
||
Name |
|
Date |
|
Exercisable |
|
Unexercisable |
|
($) |
|
Date |
|
Date |
|
(#) |
|
($) |
||
|
William J. Newell
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
3/3/2023 |
(1) (3) |
|
- |
|
10,444 |
|
5.79 |
|
3/3/2033 |
|
|
|
|
|
|
|
|
|
3/3/2023 |
(1) (3) |
|
23,437 |
|
91,119 |
|
5.79 |
|
3/3/2033 |
|
|
|
|
|
|
|
|
|
3/4/2022 |
(1) (3) |
|
- |
|
10,377 |
|
8.17 |
|
3/3/2032 |
|
|
|
|
|
|
|
|
|
3/4/2022 |
(1) (3) |
|
72,625 |
|
82,998 |
|
8.17 |
|
3/3/2032 |
|
|
|
|
|
|
|
|
|
3/5/2021 |
(1) (3) |
|
- |
|
8,228 |
|
21.11 |
|
3/4/2031 |
|
|
|
|
|
|
|
|
|
3/5/2021 |
(1) (3) |
|
137,500 |
|
54,272 |
|
21.11 |
|
3/4/2031 |
|
|
|
|
|
|
|
|
|
1/29/2020 |
(1)(3) |
|
309 |
|
2,605 |
|
10.09 |
|
1/28/2030 |
|
|
|
|
|
|
|
|
|
1/29/2020 |
(1)(3) |
|
122,086 |
|
- |
|
10.09 |
|
1/28/2030 |
|
|
|
|
|
|
|
|
|
1/29/2019 |
(1)(3) |
|
9,271 |
|
- |
|
10.45 |
|
1/28/2029 |
|
|
|
|
|
|
|
|
|
1/29/2019 |
(1)(3) |
|
435,729 |
|
- |
|
10.45 |
|
1/28/2029 |
|
|
|
|
|
|
|
|
|
9/26/2018 |
(1)(3) |
|
33,330 |
|
- |
|
15.00 |
|
9/25/2028 |
|
|
|
|
|
|
|
|
|
9/26/2018 |
(1)(3) |
|
421,215 |
|
- |
|
15.00 |
|
9/25/2028 |
|
|
|
|
|
|
|
|
|
9/28/2015 |
(2) |
|
8,347 |
|
- |
|
11.98 |
|
9/27/2025 |
|
|
|
|
|
|
|
|
|
9/28/2015 |
(2) |
|
50,717 |
|
- |
|
11.98 |
|
9/27/2025 |
|
|
|
|
|
|
|
|
|
9/28/2015 |
(2) |
|
13,774 |
|
- |
|
11.98 |
|
9/27/2025 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
3/3/2023 |
(1)(4) |
|
95,000 |
|
407,550 |
|
|
|
|
|
|
|
|
|
|
|
|
|
3/4/2022 |
(1)(4) |
|
93,375 |
|
400,579 |
|
|
|
|
|
|
|
|
|
|
|
|
|
3/5/2021 |
(1)(4) |
|
37,500 |
|
160,875 |
|
|
|
|
|
|
|
|
|
|
|
|
|
1/29/2020 |
(1)(4) |
|
13,750 |
|
58,988 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Anne Borgman, M.D. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
3/15/2023 |
(5) (3) |
|
- |
|
175,000 |
|
5.40 |
|
3/15/2033 |
|
3/15/2023 |
(5)(4) |
|
150,000 |
|
643,500 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Hans-Peter Gerber, Ph.D.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
9/18/2023 |
(5) (3) |
|
- |
|
175,000 |
|
3.77 |
|
9/18/2033 |
|
9/18/2023 |
(5)(4) |
|
150,000 |
|
643,500 |
36
37
PAY VERSUS PERFORMANCE
In accordance with rules adopted by the Securities and Exchange Commission pursuant to Section 953(a) of the Dodd-Frank Wall Street Reform and Consumer Protection Act of 2010, we provide the following disclosure regarding executive compensation for our principal executive officer (“PEO”) and our other NEOs (“Other NEOs”) and Company performance for the fiscal years listed below. The Compensation Committee did not consider the pay versus performance disclosure below in making its pay decisions for any of the years shown. The amounts shown for Compensation Actually Paid have been calculated in accordance with Item 402(v) of Regulation S-K and do not reflect compensation actually earned, realized, or received by the Company’s NEOs; these amounts reflect the Summary Compensation Table total with certain adjustments as described in the following table and footnotes.
Year |
|
Summary Compensation Table Total for CEO |
|
Compensation Actually Paid to CEO(1) |
|
Average Summary Compensation Paid to Other NEOs(2) |
|
Average Compensation Actually Paid to Other NEOs(1)(2) |
|
Value of Initial $100 Investment Based on Sutro TSR |
|
Net Income ($M) |
2023 |
|
$ |
|
$( |
|
$ |
|
$ |
|
$ |
|
$( |
2022 |
|
|
( |
|
|
|
|
|
|
|
( |
|
2021 |
|
|
|
|
|
|
( |
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
(1) To calculate Compensation Actually Paid (CAP), the following amounts were deducted from and added to the applicable SCT total compensation:
|
|
|
CEO |
||
|
|
|
2021 |
2022 |
2023 |
|
Summary Compensation Table Total |
|
$ |
$ |
$ |
|
Less: |
Grant Date Fair Value of Stock and Option Awards in the Covered Year |
|
-$ |
- |
- |
|
Plus: |
Fair Value at Year-End of Unvested Stock and Option Awards Granted in the Covered Year* |
|
+ |
+ |
+ |
|
Plus: |
Fair Value of Stock and Option Awards Granted in the Covered Year that Vested in the Covered Year* |
|
+ |
+ |
+ |
|
Change in Fair Value of Unvested Stock and Option Awards Granted in Prior Years* |
|
- |
- |
- |
|
Change in Fair Value of Stock and Option Awards from Prior Years that Vested in the Covered Year* |
|
- |
- |
- |
|
= |
Compensation Actually Paid |
|
$ |
-$ |
-$ |
|
|
|
|
|
|
|
|
|
Average of Other NEOs |
||
|
|
|
2021 |
2022 |
2023 |
|
Summary Compensation Table Total |
|
$ |
$ |
$ |
|
Less: |
Grant Date Fair Value of Stock and Option Awards in the Covered Year |
|
- |
- |
- |
Plus: |
Fair Value at Year-End of Unvested Stock and Option Awards Granted in the Covered Year* |
|
+ |
+ |
+ |
Plus: |
Fair Value of Stock and Option Awards Granted in the Covered Year that Vested in the Covered Year* |
|
+ |
+ |
|
|
Change in Fair Value of Unvested Stock and Option Awards Granted in Prior Years* |
|
- |
- |
|
|
Change in Fair Value of Stock and Option Awards from Prior Years that Vested in the Covered Year* |
|
- |
- |
|
|
= |
Compensation Actually Paid |
|
$ |
$ |
$ |
(*)
38
(2)
2023 - Anne Borgman, Hanspeter Gerber; 2022 - Trevor Hallam, Jane Chung; 2021 - Trevor Hallam, Edward Albini, Jane Chung, Arturo Molina.
The relationship between Compensation Actually Paid (CAP) and the financial performance elements reflected in the above Pay versus Performance tables are described in the below charts:
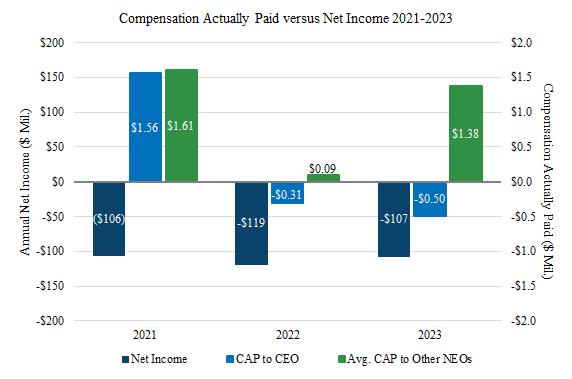
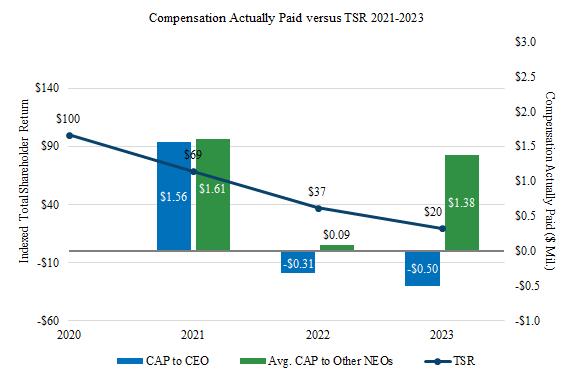
39
EQUITY COMPENSATION PLAN INFORMATION
The following table presents information as of December 31, 2023 with respect to compensation plans under which shares of our common stock may be issued.
Plan category |
|
Number of securities to be issued upon exercise of outstanding options, warrants and rights |
|
|
Weighted-average exercise price of outstanding options, warrants and rights (1) |
|
|
Number of securities remaining available for future issuance under equity compensation plans (3) |
|
|||||
Equity compensation plans approved by security holders |
|
11,797,771(2) |
|
|
$ 11.54 |
|
|
2,269,864 |
|
|||||
Equity compensation plans not approved by security holders |
|
1,479,750(4) |
|
|
8.52 |
|
|
422,966(5) |
|
|||||
Total |
|
13,277,521 |
|
|
$ 11.21 |
|
|
2,692,830 |
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
40
PROPOSAL NO. 3
ADVISORY VOTE ON EXECUTIVE COMPENSATION
In accordance with Section 14A of the Exchange Act, we are including in this proxy statement the opportunity for our stockholders to vote to approve, on a non-binding, advisory basis, the compensation of our named executive officers as disclosed in this proxy statement. This non-binding advisory vote is commonly referred to as a “say on pay” vote. We are requesting that stockholders vote, in an advisory capacity, on our named executive officer compensation as disclosed in the “Executive Compensation” section of this proxy statement at the Annual Meeting.
We strongly encourage stockholders to review the information contained in the “Executive Compensation—Compensation Disclosure” section of this proxy statement for additional details on our compensation of our Named Executive Officers, including our compensation philosophy and objectives, as well as the processes the Compensation Committee used to determine the structure and amounts of the compensation of our Named Executive Officers. The Compensation Committee and the Board believe that these policies and procedures are effective in implementing our compensation philosophy and in achieving its goals. We are asking you to indicate your support for the compensation of our Named Executive Officers as described in this Proxy Statement. This vote is not intended to address any specific item of compensation, but rather the overall compensation of our Named Executive Officers and the philosophy, policies and practices described in this Proxy Statement.
“RESOLVED, that our stockholders approve, on a non-binding advisory basis, the compensation of the named executive officers, as disclosed in the proxy statement pursuant to Item 402 of Regulation S-K, including the compensation tables and narrative discussion and the other related disclosures.”
While the results of this advisory vote are not binding, the Compensation Committee will consider the outcome of the vote in deciding whether to take any action as a result of the vote and when making future compensation decisions for our named executive officers.
OUR BOARD OF DIRECTORS RECOMMENDS A VOTE “FOR” APPROVAL OF PROPOSAL NO. 3
41
PROPOSAL NO. 4
APPROVAL OF OUR AMENDED 2018 EMPLOYEE STOCK PURCHASE PLAN
We are asking our shareholders to approve the amendment of our 2018 Employee Stock Purchase Plan (the “Amended ESPP”) to enable our employees to continue to purchase shares of our common stock under the 2018 Employee Stock Purchase Plan (the “ESPP”). The approval of this amendment of the ESPP to (i) increase the overall limit on the number of shares that may be issued under the Plan throughout its ten-year term, and (ii) make certain non-substantive clarifying revisions.
We are not asking for any additional shares for the Amended ESPP and our proposed amendment will not immediately add to or increase the pool of shares available for issuance. Rather, we are revising the overall limit on the number of shares that may be issued under the Amended ESPP throughout its ten-year term to enable use of the shares that were previously approved by our shareholders for the benefit of our employees.
In connection with our initial public offering (“IPO”) in 2018, our Board and shareholders approved the ESPP with an initial share pool of 230,000 shares, plus an automatic increase provision, or an “evergreen”, which provides that on each January 1st, through January 1, 2028, the aggregate number of shares reserved for issuance under the ESPP shall be increased by the number of shares equal to one percent (1%) of the total number of shares outstanding as of the immediately preceding December 31st (or such lesser number determined by our Board) (the resulting number of shares after accounting for each evergreen increase, the “Pool”). Because our ESPP includes an evergreen provision, we also included an overall limit on the number of shares that may be issued under the ESPP throughout its ten-year term to control the growth of the Pool that results from the evergreen increases. Upon the adoption of the ESPP in connection with the IPO, we included 2,300,000 shares as the ten-year limit (the “Original Ten-Year Limit”).
Because of the Original Ten-Year Limit, we will not be able to add shares to the Pool pursuant to our evergreen provision in 2025 absent an increase in the limit, and as a result would expect to lose the ability to utilize the ESPP in 2026. If we are no longer able to issue shares under the ESPP, our current employees would no longer enjoy this benefit, and our ability to attract and retain employees would be harmed. Accordingly, we propose to revise the Ten-Year Limit to be 3,050,000 shares (the “Revised Ten-Year Limit”).
As of April 1, 2024, a total of 2,300,000 shares have been reserved in the Pool of which 745,303 remain available for issuance. On each January 1st through January 1, 2028, the Pool will be automatically increased pursuant to the evergreen provision. We estimate that each evergreen increase will add approximately 750,000 shares to the Pool, and we will reach the Revised Ten-Year Limit in 2026. The Revised Ten-Year Limit should allow us to utilize these previously approved shares for the benefit of our current and future employees through early 2027. We are not seeking to add new shares to the Pool immediately. Rather, we are asking our shareholders to approve the Revised Ten-Year Limit so that we can continue to use the shares and exercise the evergreen provisions that were previously approved by our shareholders. The Revised Ten-Year Limit ensures that we will continue to be able to provide our eligible employees with the continuing opportunity to acquire a stock ownership interest in us through participation in a payroll deduction-based employee stock purchase plan.
The purpose of the ESPP is to provide our employees with a convenient means of acquiring an equity interest through payroll contributions, to enhance such employees’ sense of participation in our business, and to provide an incentive for continued employment through the opportunity to acquire equity at a discounted price. The Board believes that our success is due to our highly talented employee base and that our future success depends on the ability to attract and retain high caliber personnel. The Amended ESPP will be an important incentive tool supporting us in our continued efforts to attract, retain and motivate qualified personnel, while also aligning the long-term value creation objectives of our workforce with those of our shareholders.
Our headquarters is based in the San Francisco Bay Area where we must compete with many companies for a limited pool of talented people. The Board, the Compensation Committee and Company management all believe that equity compensation is essential to maintaining a balanced and competitive compensation program, has been integral to the company’s success in the past and is vital to its ability to achieve strong performance in the future.
The Amended ESPP also includes certain clarifications to remove provisions that only applied at the time of the IPO.
Vote Required and Board Recommendation
This proposal must receive a “For” vote from the holders of a majority of the shares of common stock properly casting votes for or against this proposal at the Annual Meeting in person or by proxy. If you own shares through a bank, broker or other Intermediary, you must instruct your bank, broker or other Intermediary how to vote in order for them to vote your shares so that your vote can be counted on this proposal. Abstentions and broker non-votes will not be counted toward the vote total for this proposal and therefore will not affect the outcome of this proposal.
42
OUR BOARD OF DIRECTORS RECOMMENDS A VOTE “FOR” APPROVAL OF PROPOSAL NO. 4
Description of the Amended 2018 Employee Stock Purchase Plan
The principal terms of the Amended ESPP are summarized below. This summary is qualified in its entirety by reference to the full text of the Amended ESPP, which is attached as Appendix A to this Proxy Statement.
Background
The Board and shareholders originally approved the ESPP in September 2018 and it became effective on our IPO. The Board approved the Amended ESPP on March 24, 2024 and it will become effective if approved by our shareholders at our annual meeting occurring on June 6, 2024. The Amended ESPP is intended to qualify as an “Employee Stock Purchase Plan” under the provisions of Section 421 and 423 of the Internal Revenue Code (the “Code”). The provisions of the Amended ESPP shall, accordingly, be construed so as to extend and limit participation in a manner consistent with the requirements of those sections of the Code. The Amended ESPP will not be a qualified deferred compensation plan under Section 401(a) of the Code and is not subject to the provisions of ERISA.
Administration
Our Compensation Committee will administer our ESPP subject to the terms and conditions of the Amended ESPP. Among other things, our Compensation Committee will have the authority to determine eligibility for participation in the Amended ESPP, designate separate offerings under the plan, and construe, interpret and apply the terms of the plan.
Share Reserve
We initially reserved 230,000 shares of our common stock for sale under our ESPP. The aggregate number of shares reserved for sale under our ESPP has, and will continue to, increase automatically on each January 1st through January 1, 2028 by the number of shares equal to the lesser of (i) 1% of the total outstanding shares of our common stock as of the immediately preceding December 31st (rounded to the nearest whole share) or (ii) a number of shares as may be determined by our Board in any particular year. If the Amended ESPP is approved, the aggregate number of shares issued over the term of the Amended ESPP, subject to stock-splits, recapitalizations or similar events, may not exceed 3,050,000 shares of our common stock. If the Amended ESPP is not approved, the aggregate number of shares issued over the term of our ESPP, subject to stock-splits, recapitalizations or similar events, may not exceed 2,300,000 shares of our common stock.
Offering Periods; Purchase Rights
The Amended ESPP is currently expected to provide that each offering period under the Amended ESPP is for six months and consists of one six-month purchase period, consistent with the terms of the ESPP. Offering periods under the Amended ESPP will commence on March 16th and September 16th of each year during the term of the Amended ESPP. Our Compensation Committee may change the duration and structure of future offering periods in accordance with the terms of the Amended ESPP, provided that no offering period may extend for a period longer than 27 months.
On the first trading day of each offering period (the “Offering Date”), each eligible employee who has properly enrolled in that offering period in accordance with the rules prescribed by our compensation committee will be granted an option to purchase shares of the Company’s common stock to be funded by payroll deductions, based on the participant’s elected contribution rate. Unless a participant has properly withdrawn from the offering period, each option granted under the Amended ESPP will automatically be exercised on the last trading day of each purchase period within the offering period (each, a “Purchase Date”). The purchase price will be equal to 85% of the lesser of the fair market value of our common stock on (i) the Offering Date and (ii) the Purchase Date.
Eligibility
Employees eligible to participate in any offering pursuant to the Amended ESPP generally include any employee that is employed by us or certain of our designated subsidiaries at the beginning of the offering period. However, employees who are customarily employed for 20 hours or less per week or for five months or less in a calendar year may not be eligible to participate in the Amended ESPP. In addition, any employee who owns (or is deemed to own as a result of attribution) 5% or more of the total combined voting power or value of all classes of our capital stock, or the capital stock of one of our qualifying subsidiaries, or who will own such amount as a result of participation in the Amended ESPP, will not be eligible to participate in the Amended ESPP. Our Compensation
43
Committee may impose additional restrictions on eligibility from time to time. As of April 1, 2024, all of our employees were eligible to participate in the Amended ESPP.
Enrollment in the Amended ESPP
Eligible employees become participants in the Amended ESPP by completing a subscription agreement or enrolling online, authorizing payroll deductions prior to the applicable Offering Date. A person who becomes employed after the commencement of an offering period may not participate in the Amended ESPP until the commencement of the next offering period.
Once an employee becomes a participant in an offering period, the participant will be automatically enrolled in each subsequent offering period at the same contribution level. A participant may reduce his or her contribution in accordance with procedures set forth by our Compensation Committee and may withdraw from participation in the Amended ESPP at any time prior the end of an offering period, or such other time as may be specified by our Compensation Committee. Upon withdrawal, the accumulated payroll deductions will be returned to the participant without interest.
Participation
Participating employees will be able to purchase shares of our common stock by accumulating funds through payroll deductions. Participants may select a rate of payroll deduction between 1% and 15% of their compensation, as defined in the Amended ESPP. However, a participant may not purchase more than 2,500 shares during any one purchase period (or such lower or higher number as may be determined by our Compensation Committee) and may not subscribe for more than $25,000 in fair market value of shares of our common stock (determined as of the date the offering period commences) in any calendar year in which the offering is in effect.
Adjustments upon Recapitalization
If the number of outstanding shares of our common stock is changed by a stock dividend, recapitalization, stock split, reverse stock split, subdivision, combination, reclassification, or similar change in our capital structure without consideration, then our Compensation Committee will proportionately adjust the number and class of shares available under the Amended ESPP, the purchase price and number of shares any participant has elected to purchase, as well as the maximum number of shares a participant may purchase on any one Purchase Date.
Change of Control
If we experience a change of control transaction, any offering period that commenced prior to the closing of the proposed change of control transaction will be shortened and terminated on a new purchase date. The new purchase date will occur on or prior to the closing of the proposed change of control transaction, and our Amended ESPP will then terminate on the closing of the proposed change of control.
Transferability
A participant may not assign, transfer, pledge or otherwise dispose of payroll deductions credited to his or her account, or any rights with regard to an election to purchase shares pursuant to the Amended ESPP other than by will or the laws of descent or distribution.
Amendment; Termination
The Amended ESPP will expire on the earlier of (x) the issuance of all of the shares of common stock reserved for issuance under the Amended Plan; (y) the tenth anniversary of the original effective date of the ESPP; or (z) an earlier termination of the Amended ESPP as approved by our Board. The Compensation Committee may generally amend, suspend or terminate the Amended ESPP at any time without shareholder approval, except as may be required by applicable law or exchange listing rules.
New Plan Benefits
Participation in the Amended ESPP is voluntary and each eligible employee will have the discretion to determine whether and to what extent to participate in and contribute to the Amended ESPP. Accordingly, the benefits and amounts that will be received or allocated to officers and other employees under the Amended ESPP are not determinable at this time. Non-employee directors of the Board are not eligible to participate in the Amended ESPP.
44
Historical Plan Benefits
The following table shows, as to each of the individuals or groups indicated, the aggregate number of shares of common stock purchased under the ESPP since its inception through April 1, 2024. No shares of common stock have been purchased under the ESPP by (i) any individual director nominee who is not an employee, (ii) the current non-employee directors as a group, or (iii) any associate of any of our directors (including nominees). No person received 5% or more of the total shares of common stock purchased under the ESPP since its Inception.
Aggregate Purchases under the ESPP since its inception:
|
|
|
|
|
|
|
|||
Name and Position |
|
Aggregate Number of |
|
|
|
|
|||
William J. Newell, Chief Executive Officer |
|
|
- |
|
|
|
|||
Anne Borgman, M.D., Chief Medical Officer |
|
|
- |
|
|
|
|||
Hans-Peter Gerber, Ph.D., Chief Scientific Officer |
|
|
- |
|
|
|
|||
All current executive officers as a group (7 person) |
|
|
2,500 |
|
|
|
|||
All current and former employees, excluding current executive officers as a group |
|
|
1,552,197 |
|
Certain U.S. Federal Income Tax Consequences
The following is a general summary of the United States federal income tax consequences to us and to participants in the Amended ESPP based on tax laws in effect as of the date of this Proxy Statement. This summary is not intended to be exhaustive and does not address all matters that may be relevant to any particular participant. Among other considerations, this summary does not describe the tax laws of any state, municipality or foreign jurisdiction, or describe gift, estate, excise, payroll or other employment taxes. Participants are advised to consult with their tax advisors regarding the tax consequences of participation in the Amended ESPP. The Amended ESPP is intended to qualify as an “employee stock purchase plan” under Section 423 of the Internal Revenue Code and the following discussion is based on the assumption that it is so qualified.
Each participant’s payroll deductions under the Amended ESPP will be made on an after-tax basis. Generally, the participant will not recognize any taxable income at the time he or she is granted an option to purchase shares of common stock during an offering period or at the time the option is exercised to purchase shares on behalf of the participant. The participant will generally only recognize taxable income (or loss) on the date the participant sells or otherwise disposes of the acquired shares. The particular tax consequence depends on the length of time such shares are held by the participant prior to the sale or disposition.
If the shares are sold or disposed of more than two years from the first day of the offering period during which the shares were purchased, and more than one year from the Purchase Date or if the participant dies while holding the shares, the participant (or his or her estate) will recognize ordinary income measured as the lesser of (i) the amount by which the fair market value of the shares on the Offering Date exceeded the purchase price of the shares (calculated as though the shares had been purchased on the Offering Date) and (ii) the excess of the fair market value of the shares at the time of such sale or other disposition over the purchase price. Any additional gain will be treated as long-term capital gain. If the shares are held for the holding periods described above but are sold for a price that is less than the purchase price, there is no ordinary income and the participating employee has a long-term capital loss for the difference between the sale price and the purchase price. If the shares are sold or otherwise disposed of before the expiration of either of the holding periods described above, the participant will recognize ordinary income generally measured as the excess of the fair market value of the shares on the date the shares are purchased over the purchase price. Any additional gain or loss on such sale or disposition will be long-term or short-term capital gain or loss, depending on how long the shares were held following the date they were purchased by the participant prior to disposing of them.
We are generally not entitled to a deduction for amounts taxed as ordinary income or capital gain to a participant except to the extent of ordinary income recognized upon a sale or disposition of shares prior to the expiration of the holding periods described above.
45
From January 1, 2022 to the present, there have been no transactions, and there are currently no proposed transactions, in which the amount involved exceeds the lesser of $120,000 and 1.0% of the average of our total assets during the last two completed fiscal years to which we or any of our subsidiaries was (or is to be) a party and in which any director, director nominee, executive officer, holder of more than 5% of our capital stock, or any immediate family member of or person sharing the household with any of these individuals, had (or will have) a direct or indirect material interest, except for payments set forth under “Proposal No. 1 Election of Class III Directors” and “Executive Compensation” above.
Policies and Procedures for Related-Person Transactions
Our Board of Directors has adopted a written related-person transactions policy. Under this policy, our executive officers, directors, nominees for election as a director, beneficial owners of more than 5% of our common stock, and any members of the immediate family of and any entity affiliated with any of the foregoing persons, are not permitted to enter into a material related-person transaction with us without the review and approval of our Audit Committee, or a Committee composed solely of independent directors in the event it is inappropriate for our Audit Committee to review such transaction due to a conflict of interest. The policy provides that any request for us to enter into a transaction with an executive officer, director, nominee for election as a director, beneficial owner of more than 5% of our common stock or with any of their immediate family members or affiliates in which the amount involved exceeds $120,000 will be presented to our Audit Committee for review, consideration and approval. In approving or rejecting any such proposal, our Audit Committee will consider the relevant facts and circumstances available and deemed relevant to the Audit Committee, including, but not limited to, whether the transaction is on terms no less favorable than terms generally available to an unaffiliated third party under the same or similar circumstances and the extent of the related person’s interest in the transaction.
46
ADDITIONAL INFORMATION
Stockholder Proposals to be Presented at Next Annual Meeting
Requirements for Stockholder Proposals to be Brought Before an Annual Meeting. Our Amended and Restated Bylaws provide that for stockholder nominations to our Board of Directors or other proposals to be considered at an annual meeting of stockholders, the stockholder must give timely notice thereof in writing to the Corporate Secretary at Sutro Biopharma, Inc., 111 Oyster Point Boulevard, South San Francisco, California, 94080.
To be timely for our company’s annual meeting of stockholders to be held in 2025 (2025 Annual Meeting), a stockholder’s notice must be delivered to or mailed and received by our Corporate Secretary at our principal executive offices not earlier than 5:00 p.m. Eastern Time on February 6, 2024, and not later than 5:00 p.m. Eastern Time on March 8, 2024. A stockholder’s notice to the Corporate Secretary must set forth as to each matter the stockholder proposes to bring before the 2025 Annual Meeting the information required by applicable law and our Amended and Restated Bylaws. However, if the date of the 2025 Annual Meeting is more than 30 days before or more than 70 days after the one-year anniversary of the date of our 2024 Annual Meeting, for the stockholder notice to be timely, it must be delivered to the Corporate Secretary at our principal executive offices not earlier than the close of business on the 120th day prior to the currently proposed annual meeting and not later than the close of business on the later of (1) the 90th day prior to such annual meeting or (2) the close of business on the 10th day following the day on which public announcement of the date of such meeting is first made by us. To comply with our Amended and Restated Bylaws as well as the universal proxy rules, stockholders who intend to solicit proxies in support of director nominees other than our nominees for 2025 Annual Meeting must ensure that our Corporate Secretary receives written notice that sets forth all information required by our Amended and Restated Bylaws and by Rule 14a-19(b) under the Exchange Act within the time frames set forth above.
Requirements for Stockholder Proposals to be Considered for Inclusion in our Proxy Materials. Stockholder proposals submitted pursuant to Rule 14a-8 under the Exchange Act and intended to be presented at our 2025 Annual Meeting must be received by us not later than December 27, 2024, in order to be considered for inclusion in our proxy materials for that meeting. A stockholder’s notice to the Corporate Secretary must set forth as to each matter the stockholder proposes to bring before the 2025 Annual Meeting the information required by applicable law and our Amended and Restated Bylaws.
Available Information
The Annual Report on Form 10-K is also available at https://ir.sutrobio.com/.
“Householding” – Stockholders Sharing the Same Address
The SEC has adopted rules that permit companies and intermediaries (such as brokers) to implement a delivery procedure called “householding.” Under this procedure, multiple stockholders who reside at the same address may receive a single copy of our Annual Report on Form 10-K and proxy materials, including the Notice of Internet Availability, unless the affected stockholder has provided other instructions. This procedure reduces printing costs and postage fees, and helps protect the environment as well.
We expect that a number of brokers with account holders who are our stockholders will be “householding” our Annual Report on Form 10-K and proxy materials, including the Notice of Internet Availability. A single Notice of Internet Availability and, if applicable, a single set of our Annual Report on Form 10-K and other proxy materials will be delivered to multiple stockholders sharing an address unless contrary instructions have been received from one or more of the affected stockholders. Once you have received notice from your broker that it will be “householding” communications to your address, “householding” will continue until you are notified otherwise or until you revoke your consent. Stockholders may revoke their consent at any time by contacting their broker. Stockholders may revoke their consent at any time by contacting Equiniti Trust Company LLC, either by calling toll-free (800) 937-5449, or by writing to Equiniti Trust Company LLC Operations Center, 6201 15th Avenue, Brooklyn, New York 11219.
47
Upon written or oral request, we will undertake to promptly deliver a separate copy of the Notice of Internet Availability and, if applicable, Annual Report on Form 10-K and other proxy materials to any stockholder at a shared address to which a single copy of any of those documents was delivered. To receive a separate copy of the Notice of Internet Availability and, if applicable, Annual Report on Form 10-K and other proxy materials, you may write our Investor Relations Department at Sutro Biopharma, Inc., 111 Oyster Point Boulevard, South San Francisco, California, 94080, Attn: Investor Relations, or at IR@sutrobio.com.
Any stockholders who share the same address and currently receive multiple copies of our Notice of Internet Availability or Annual Report on Form 10-K and other proxy materials who wish to receive only one copy in the future can contact their bank, broker or other holder of record to request information about “householding” or our Investor Relations Department at the address or telephone number listed above.
OTHER MATTERS
Our Board of Directors does not presently intend to bring any other business before the meeting and, so far as is known to the Board of Directors, no matters are to be brought before the meeting except as specified in the notice of the meeting. As to any business that may arise and properly come before the meeting, however, it is intended that proxies, in the form enclosed, will be voted in respect thereof in accordance with the judgment of the persons voting such proxies.
48
APPENDIX A
Amended 2018 Employee Stock Purchase Plan
SUTRO BIOPHARMA, INC.
AMENDED 2018 EMPLOYEE STOCK PURCHASE PLAN
Subject to Section 14, a total of Two Hundred Thirty Thousand (230,000) Shares is reserved for issuance under this Plan. In addition, on each January 1 for the first ten (10) calendar years after the Effective Date, the aggregate number of Shares reserved for issuance under the Plan shall be increased automatically by the number of Shares equal to one percent (1%) of the total number of outstanding Shares outstanding on the immediately preceding December 31 (rounded down to the nearest whole share); provided, that the Board or the Committee may in its sole discretion reduce the amount of the increase in any particular year. Subject to Section 14, no more than Three Million Fifty Thousand (3,050,000) Shares may be issued over the term of this Plan. The number of Shares initially reserved for issuance under this Plan and the maximum number of Shares that may be issued under this Plan shall be subject to adjustments effected in accordance with Section 14.
49
The foregoing notwithstanding, an individual shall not be eligible if his or her participation in the Plan is prohibited by the law of any country that has jurisdiction over him or her, if complying with the laws of the applicable country would cause the Plan to violate Section 423 of the Code, or if he or she is subject to a collective bargaining agreement that does not provide for participation in the Plan.
50
51
52
(i) In the case of Shares purchased during an Offering Period that commenced in the current calendar year, the limit shall be equal to (A) $25,000 minus (B) the Fair Market Value of the Shares that the Participant previously purchased in the current calendar year (under this Plan and all other employee stock purchase plans of the Company or any parent or Subsidiary of the Company).
(ii) In the case of Shares purchased during an Offering Period that commenced in the immediately preceding calendar year, the limit shall be equal to (A) $50,000 minus (B) the Fair Market Value of the Shares that the Participant previously purchased (under this Plan and all other employee stock purchase plans of the Company or any parent or Subsidiary of the Company) in the current calendar year and in the immediately preceding calendar year.
For purposes of this Subsection (a), the Fair Market Value of Shares shall be determined in each case as of the beginning of the Offering Period in which such Shares are purchased. Employee stock purchase plans not described in Section 423 of the Code shall be disregarded. If a Participant is precluded by this Subsection (a) from purchasing additional Shares under the Plan, then his or her employee contributions shall automatically be discontinued and shall automatically resume at the beginning of the earliest Purchase Period that will end in the next calendar year (if he or she then is an eligible employee), provided that when the Company automatically resumes such payroll deductions, the Company must apply the rate in effect immediately prior to such suspension.
53
54
55
56
57
58
59
60

SUTRO BIOPHARMA SUTRO BIOPHARMA, INC. 111 OYSTER POINT BOULEVARD SOUTH SAN FRANCISCO, CALIFORNIA 94080 SCAN TO VIEW MATERIALS & VOTE VOTE BY INTERNET - www.proxyvote.com or scan the QR Barcode above Use the Internet to transmit your voting instructions and for electronic delivery of information. Vote by 11:59 P.M. ET on 06/07/2023. Have your proxy card in hand when you access the web site and follow the instructions to obtain your records and to create an electronic voting instruction form. During The Meeting - Go to www.virtualshareholdermeeting.com/STRO2023 You may attend the meeting via the Internet and vote during the meeting. Have the information that is printed in the box marked by the arrow available and follow the instructions. VOTE BY PHONE - 1-800-690-6903 Use any touch-tone telephone to transmit your voting instructions. Vote by 11:59 P.M. ET on 06/07/2023. Have your proxy card in hand when you call and then follow the instructions. VOTE BY MAIL Mark, sign and date your proxy card and return it in the postage-paid envelope we have provided or return it to Vote Processing, c/o Broadridge, 51 Mercedes Way, Edgewood, NY 11717. TO VOTE, MARK BLOCKS BELOW IN BLUE OR BLACK INK AS FOLLOWS: KEEP THIS PORTION FOR YOUR RECORDS DETACH AND RETURN THIS PORTION ONLY THIS PROXY CARD IS VALID ONLY WHEN SIGNED AND DATED. The Board of Directors recommends you vote FOR the following: For All Withhold All For All Except To withhold authority to vote for any individual nominee(s), mark “For All Except” and write the number(s) of the nominee(s) on the line below. 1. Election of Directors Nominees 01) William J. Newell 02) Connie Matsui 03) James Panek The Board of Directors recommends you vote FOR proposals 2, 3 and 4. For Against Abstain 2. To ratify the appointment of Ernst & Young LLP as our independent registered public accounting firm for the fiscal year ending December 31, 2023. 3. To approve, on a non-binding, advisory basis, the compensation of our named executive officers. 4. To approve the amendment and restatement of our restated certificate of incorporation to permit the exculpation of our officers from personal liability for certain breaches of the duty of care. NOTE: Such other business as may properly come before the meeting or any adjournment thereof Please sign exactly as your name(s) appear(s) hereon. When signing as attorney, executor, administrator, or other fiduciary, please give full title as such. Joint owners should each sign personally. All holders must sign. If a corporation or partnership, please sign in full corporate or partnership name by authorized officer. Signature [PLEASE SIGN WITHIN BOX] Date Signature (Joint Owners) Date 0000609047_1 R1.0.0.6
61

Important Notice Regarding the Availability of Proxy Materials for the Annual Meeting: The Notice and Proxy Statement and Form 10-K are available at www.proxyvote.com SUTRO BIOPHARMA, INC. Annual Meeting of Shareholders June 8, 2023 12:00 PM PT This proxy is solicited by the Board of Directors The undersigned hereby appoints William J. Newell and Edward C. Albini, and each of them, with full power of substitution and power to act alone, as proxies to vote all the shares of Common Stock which the undersigned would be entitled to vote if personally present and acting at the Annual Meeting of Shareholders of SUTRO BIOPHARMA, INC., to be held on June 8, 2023, via a live webcast at www.virtualshareholdermeeting.com/STRO2023, and at any adjournments or postponements thereof. This proxy, when properly executed, will be voted in the manner directed herein. If no such direction is made, this proxy will be voted FOR ALL NOMINEES in Proposal 1, FOR Proposal 2, FOR Proposal 3 and FOR Proposal 4. Continued and to be signed on reverse side 0000609047_2 R1.0.0.6
62
