DRSLTR: Correspondence Related to Draft Registration Statement
Published on August 20, 2018

|
||||
| August 20, 2018 | ||||
| ROBERT A. FREEDMAN | EMAIL: RFREEDMAN@FENWICK.COM Direct Dial: +1 (650) 335-7292 |
|||
VIA EDGAR AND OVERNIGHT DELIVERY
U.S. Securities and Exchange Commission
Division of Corporation Finance
100 F Street, NE
Washington, DC 20549
| Attention: | John Reynolds, Assistant Director | |||
| Irene Barberena-Meissner, Staff Attorney | ||||
| Kevin Dougherty, Staff Attorney | ||||
| Ethan Horowitz, Accounting Branch Chief | ||||
| Wei Lu, Staff Accountant | ||||
| Re: | Sutro Biopharma, Inc. | |||
| Amendment No. 1 to Draft Registration Statement on Form S-1 | ||||
| Submitted July 10, 2018 | ||||
| CIK No. 0001382101 | ||||
Ladies and Gentlemen:
On behalf of Sutro Biopharma, Inc. (the Company), we are providing the staff (the Staff) of the Securities and Exchange Commission (the Commission) with the Companys proposed revisions to the chart included on pages 2 and 110 of the Registration Statement, as discussed with the Staff by telephone conference on August 17, 2018.
The Company advises the Staff that it intends to divide the original chart included in the Registration Statement into two separate and distinct charts, as shown below. The first chart will be a pipeline chart and include the Companys two internal clinical and near-clinical stage product candidates and its two collaboration programs for which Investigational New Drug applications are expected to be filed in the near term. The second chart will be a discovery program/preclinical chart and include the Companys internal and partnered programs that are in discovery or preclinical development stages. This discovery/preclinical chart will include its own title, as shown below, that makes it clear that these programs are in earlier stages of development and may never advance to clinical development or product candidates.
Additionally, in order to further emphasize the risks and uncertainty associated with programs in the Companys discovery/preclinical chart, the Company advises the Staff that it intends to include additional disclosure in the risk factor section of the Registration Statement regarding the challenges of clinical development, including that the Companys programs listed in the discovery/preclinical chart are in earlier stages of development and may never advance to clinical-stage development.
U.S. Securities and Exchange Commission
August 20, 2018
Page 2
The following is the Companys proposed revised presentation of its pipeline and discovery/preclinical-stage programs.
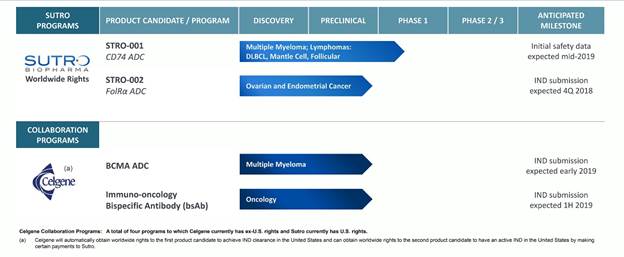
Our IND-Enabling and Clinical-Stage Product Candidates
Our current product candidates, all based on our proprietary XpressCF Platform, are summarized in the chart below:
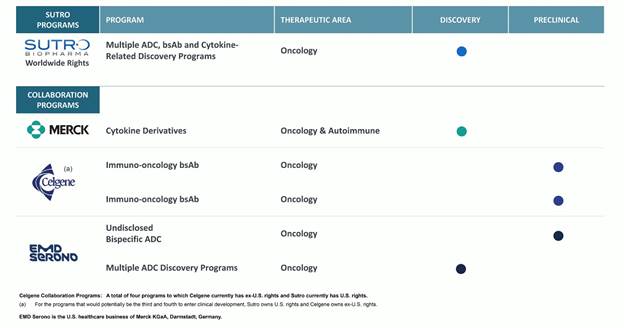
Our Discovery and Preclinical Programs
We and our collaborators are actively pursuing the discovery and development of other novel cytokine derivatives, ADCs and bispecifics, as summarized in the chart below.
U.S. Securities and Exchange Commission
August 20, 2018
Page 3
* * * * * * *
Should the Staff have additional questions or comments regarding the foregoing, please do not hesitate to contact the undersigned at (650) 335-7292, or, in his absence, Amanda Rose at (206) 389-4553.
| Sincerely, |
| FENWICK & WEST LLP |
| /s/ Robert A. Freedman |
| Robert A. Freedman |
| Partner |
| cc: | William J. Newell, Chief Executive Officer |
| Edward Albini, Chief Financial Officer |
| Sutro Biopharma, Inc. |
| Amanda L. Rose |
| Fenwick & West LLP |
| David Peinsipp |
| Charles S. Kim |
| Andrew S. Williamson |
| Cooley LLP |